Cosmetri Product Manager
11 Reviews 5/5 ★ ★ ★ ★ ★A cloud-based cosmetic manufacturing software with US FDA +Prop65 compliance, R&D formulation, and production tools.
Product Overview
Cosmetri Product Manager software aims to help cosmetics and personal care companies by streamlining operations, accelerating product development, and ensuring compliance with strict regulations. Check regulations for over 55 countries, including US FDA (+ Prop65), Canada, EU, ASEAN and Mercosur. The fully scalable, cloud-based software can be configured as an enterprise platform to maximize the effectiveness of teams and provide all staff members and roles with the tools required to perform their tasks.Pros
- Advanced R&D/formulation tools
- Complies with regulations across over 55 countries
- Template-based reporting builder
Cons
- Short learning curve to utilize software
- Cannot load raw materials on an order
- Exact pricing details not provided by the developer
Target Market
Businesses operating in the cosmetics sector, particularly those engaged in product development, formulation, and quality assurance.Video Overview
Features
The features of Cosmetri Product Manager include:
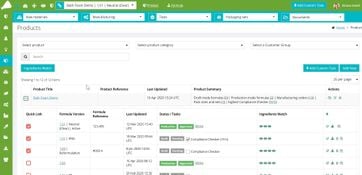
R&D/Formulation
- Easy-to-use tools for professional cosmetics formulators.
- Integrated sample development QA and inventory management for R&D samples.
- Approve and lock a formula and all related items.
- Advanced template-based reporting builder.
Testing/QA
- QA checklists ensure adherence to standard operating procedures with full traceability.
- Require test conformity to streamline processes and reduce errors.
- Easy overview of test schedules, including stability testing.
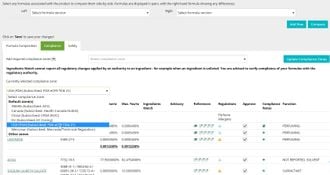
Compliance
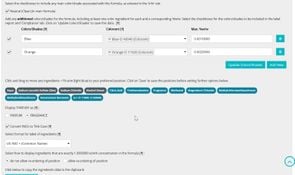
- View the formula’s INCI breakdown by %w/w, including the calculation of fragrance allergens and additional color pigments.
- Manage compliance of the formula by countries/markets. Build advisory lists for additional policies.
- Be alerted to changes by an authority regarding any ingredient in your formula.
MRP
- Keep track of your raw material and packaging inventory.
- Reserve inventory for lab use and split batches between locations and customers.
- Raw materials required for planned production
- Full QA management of received materials.
- MRP reporting and inventory stock take.
- Dispense inventory according to FIFO.
Production
- Manage production orders with integrated quality control and testing, including SOP checklists.
- Separate production workflow for sample development.
- Ensure full traceability of all produced batches, including dispense lists, batch tickets, and test reports.
Packaging
- Build packaging sets with a single-click pack-out function.
- Test packaging along with formula samples.
- Full packaging inventory management, including MRP and dispense lists.
- Calculate and optimize packaging costs per SKU.
Pricing Plans
Cosmetri Product Manager can be purchased in 3 different plans, including:
- Essentials: Includes 3 users (5 max) and 100 products
- Standard: Includes 4 users (10 max) and unlimited products.
- Professional: Customized for larger teams with over 10 users. All plans require businesses to request a quote for customized pricing.
Product Overview
Developer Overview
User Reviews of Cosmetri Product Manager
Write a Review
- Consumer Goods
- 11-50 employees
- Annual revenue $1M-$10M
Cosmetri Product Manager Review
Support team responds quickly and is very easy to work with it. It is great with quick responses.
Pros
Easy to have multiple versions of formulas.
Cons
Would like a way to load raw materials that are on order.

- Consumer Goods
- 1-10 employees
- Annual revenue $1M-$10M
An outstanding tool for cosmetic formulators and manufacturers
An outstanding tool for cosmetic formulators and manufacturers
Pros
Cosmetri allows me to collate all of my formulation, ingredient, and compliance information in one location which can be accessed readily. It has become an indispensable tool in my work. Cosmetri allows me to remain highly organized and ensure that I am meeting compliance requirements.
Cons
There is a short learning curve for use of the software - this is to be expected and is not a criticism.
- Consumer Goods
- 51-250 employees
- Annual revenue $10M-$50M
This is a big step for our company
This is a big step for our company as we have outgrown our old archaic method of generating formulas in Microsoft Excel and using non-industry specific manufacturing software.
Pros
I've been amazed about how quickly I can enter formulas in to cosmetri. It all just makes sense!

- Consumer Goods
- 1-10 employees
- Annual revenue $0-$1M
We use every function of Cosmetri, and this software is at the heart of our business
We started our business three years ago and began using Cosmetri immediately. I was so happy that we found a software that covered all our needs. From formulation planning, PIF generation to safety data analyses and calculations (SEM, MoS etc).
Pros
Without this powerful tool it would have been impossible to fulfill all the compliance and regulation tasks in reasonable time. Nowadays, we use every function of Cosmetri, and this software is at the heart of our business. Last month we realized our ISO:22716 certification. Cosmetri and all the documentation provided was a real big help.

- Consumer Goods
- 1-10 employees
- Annual revenue $0-$1M
cosmetri is superior to all of them
What I love about cosmetri is how easy it makes R&D work. I can easily create stability testing programmes and manage multiple formulations. It basically does the work of one employee.
Pros
Having used other similar R&D software, cosmetri is superior to all of them due to its high functionality and ease of managing large amounts of data.
- Consumer Goods
- 1-10 employees
- Annual revenue $0-$1M
Its ease of use in and around batch management
Cosmetri is without doubt the most comprehensive and user friendly compliance software for our industry. We used it to obtain and maintain our GMP ISO22716. From the management of raw materials to final batch protocols the system just delivers. We are now looking at implementing the GMP module to Manage the full scope of our certification.
Pros
Its ease of use in and around batch management.
Cons
I still have to run an MRP system that has a high level of duplication across the two. If cosmetri could raise a purchase order then allow me to receipt those materials in i could remove 70% of my duplication.
- Professional Services
- 1-10 employees
- Annual revenue $0-$1M
A one-stop shop for Cosmetic Product Development
A one-stop shop for Cosmetic Product Development
Pros
Up-to-date information about chemical ingredients, regulatory aspects separated for different regions
Cons
Some information difficult to find, limitations for special characters (- etc., limited storage for products
- Consumer Goods
- 1-10 employees
- Annual revenue $0-$1M
A all in one software solution to make sure you and your company comply
A all in one software solution to make sure you and your company comply with the newest current ISO22716 Cosmetic GMP.
Pros
Being a small company I don't have the benefit of having a large organization with alot of knowledge and know how. Cosmetri GMP makes it possible for us as a small company to comply in an easy way, and to be confident that our products stay within current required regulation.
Cons
As a creative I hate numbers in general. But if I didn't have Cosmetri GMP it would take me 3-4 times as much admin time to make sure I comply. But the feature where the software constantly looks over your ingredients to make sure you stay compliant is ingenious. It makes sure I sleep well at night!
- Consumer Goods
- 1-10 employees
- Annual revenue $0-$1M
It makes the whole process of developing a product incredibly easy
This is an amazing software that makes it easy to setup your formulas, PIF and make sure that your formulas stay compliant with the Current regulatory. You will get notifications if it don’t! Giving you security and the confidence you need when your “babies” leave your house!
Pros
It makes the whole process of developing a product incredibly easy. No need for papers. All is conducted inside Cosmetri. It is a walk through from product formulating to finished product. Evaluations of current packaging and a powerful tool to make your team work tidy and towards the main goal.
Cons
At the moment I have no bad experiences with Cosmetri. I am not a cosmetic chemist, but with this software and with the help from my Safety Assessor I have all I need to streamline a product line.
- Internet Software & Services
- 1-10 employees
- Annual revenue $0-$1M
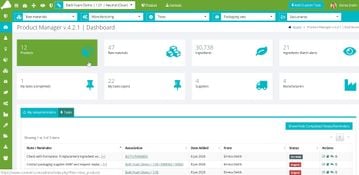
Access the tools you need from one integrated dashboard
cosmetri Product Manager is the only PLM software for cosmetics businesses, providing tools for all stages of product lifecycle - from R&D/formulation to compliance management, sample development, manufacturing, testing/QA and manufacturing/MRP.
Pros
Access the tools you need from one integrated dashboard. Advanced regulatory checking including US FDA, Canada and Prop65. Easy to follow user guides, in-application help, and online training.
Cons
No multi-language version yet available.

- Internet Software & Services
- 1-10 employees
- Annual revenue $0-$1M
Our existing customers love the simplicity
Intended specifically for cosmetics manufacturers, cosmetri GMP significantly reduces the complexity of complying with GMP ISO 22716. Fast and easy implementation means that you can be fully operational within 2-3 days. Online training included. No setup fees.
Pros
Our existing customers love the simplicity, fast implementation and 'all in one' management of GMP tasks.
Cons
Full integration with cosmetri Product Manager is not yet completed.