Intellect QMS
A quality management software to help ensure compliance with FDA and ISO regulations for mid to large enterprises.
Product Overview
Intellect QMS is a quality management software that helps leaders meet FDA, ISO, and other global GxP regulatory compliance requirements. It provides onsite and remote workforce with virtual auditing, online approvals, 21 CFR Part 11 electronic signatures. It ensures teams can access only approved and up-to-date quality documents, data, reports and analytics anytime, on any device.
The no-code platform allows users to create and configure applications without needing specialized programming skills.
Pros
- Easy to implement
- On-premise and Cloud
- Support for ISO standards and FDA regulations
Cons
- Requires a quote for pricing details
- Initial training can be complex
Target Market
Mid-size to large enterprises, particularly in the pharmaceuticals, healthcare, and manufacturing industries.Not Recommended For
Very small businesses or those seeking a ready-to-use, out-of-the-box solution with minimal customization needs.Video Overview
Intellect QMS Features
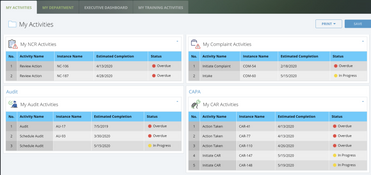
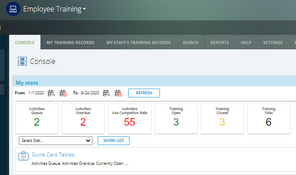
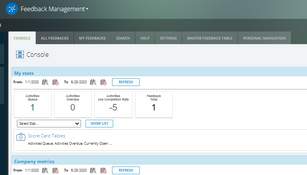
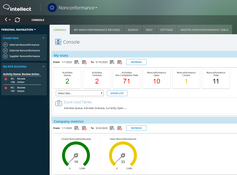
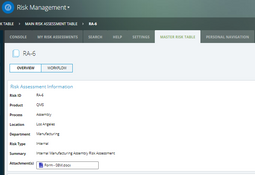
The system offers over 30 applications for over 9 ISO standards. These include:
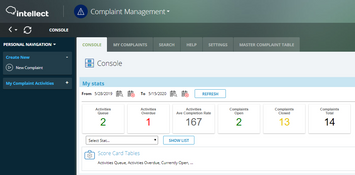
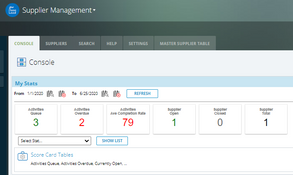
- Dashboards
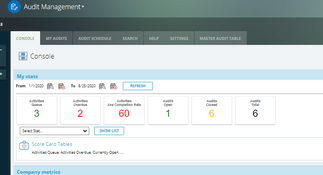
- Audit management
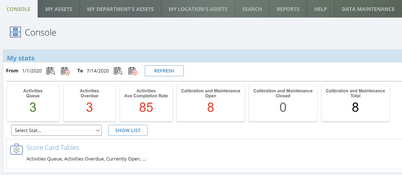
- Calibration and maintenance
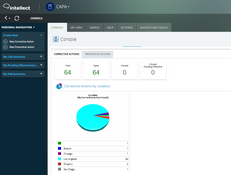
- CAPA
- FDA, EU, and OSHA regulations
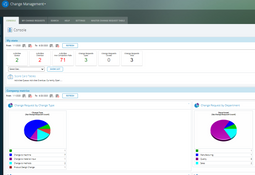
- Change management
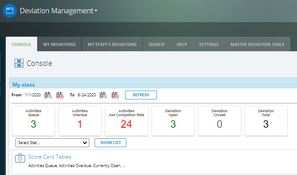
- Deviation management
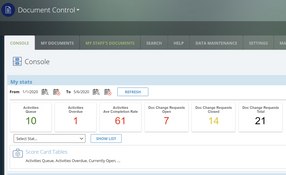
- Document control
- Employee training
- Nonconformance
- Risk management
- Supplier management
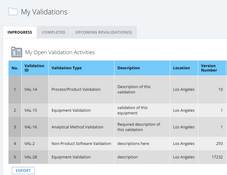
- Validation and verification
- Mobile app
Intellect AI
Intellect launched its own proprietary AI that’s integrated with OpenAI. One of the standout functions is the AI Smart Analysis, which lets you engage in plain-language conversations to extract insights on data trends, root causes, and recommended solutions to problems. It’s basically a built-in ChatGPT that uses your QMS data. While it’s not a revolutionary addition to the system, it can help you find some data faster.
Industries
- General Manufacturing
- Pharmaceuticals & Biotech
- Life sciences
- Laboratories
Pricing Plans
Intellect QMS does not disclose pricing, but its tiered plans include essentials, professional, and enterprise with increased storage space and core features per plan. Businesses must contact Intellect QMS to receive a customized quote. Important factors include:
- Number of users
- Industry and add-ons
- Required customizations
- Services like data migration or extended training
- Deployment preference (AWS, AWS GovCloud, AWS HIPAA)
Product Overview
Developer Overview
Related Products
User Reviews of Intellect QMS
No reviews have been submitted. Do you use Intellect QMS? Have you considered it as part of your software evaluation process? Share your perspective by writing a review, and help other organizations like yours make smarter, more informed software selection decisions!