Qualcy eQMS Review: Features, Ratings
We like Qualcy’s modular structure, customizable user permissions, and transparent pricing model. It publicly publishes pricing details, unlike most QMS. Qualcy starts at $399 a month with a free 30-day trial.
However, it’s not for companies needing strong in-app guidance or support in the natural resource sector. In addition, users should prepare to invest time upfront in setting up user roles and establishing initial training and risk management plans within the system.
- Transparent pricing and free trial
- Role-based access control
- Scalable, modular architecture
- Not a great fit for oil and gas
- Limited in-app support
- Lengthy setup process
- Developer Qualcy Systems Inc
- Client OS Web
- Deployment Cloud or On-Premises
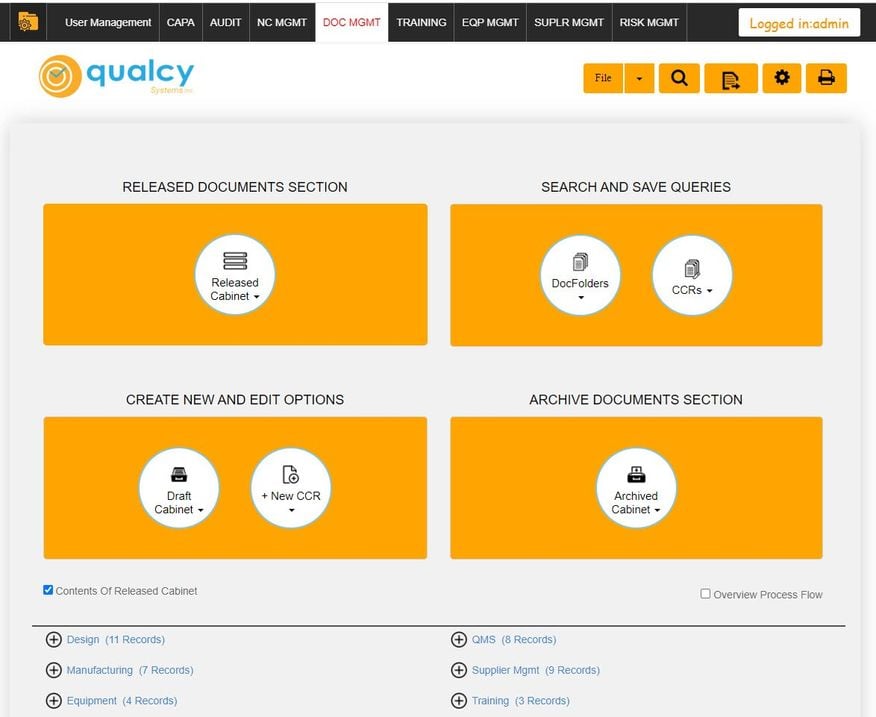
What is Qualcy eQMS?
This web-based QMS software aids in document, training records, supplier quality, and equipment management. Additional functionalities include CAPA, non-conforming material reporting, CMMS, calibration, and audit capabilities.
Qualcy offers full compliance with 21 CFR 820, 210, and 211 requirements and assists with maintaining ISO 13485 standards. We sat down with the President of Qualcy Systems to review Qualcy eQMS.
Our Ratings
| Usability - 7 | Could improve user interface and in-app guidance, but system is designed for ease of use, allowing users to find records by status; setting up user role permissions and access controls is time-consuming but reduces user input after initial setup. |
| Support - 7 | Basic plan offers phone support and online training; users must upgrade to gold or platinum plans for webinars, QMS consulting, etc.; system provides a built-in Help File section with documents and videos provided by Qualcy or the organization. |
| Scalability - 10 | Offers modules that users can purchase separately; customizable user permissions allow for control and flexibility as teams grow; Qualcy Systems plans to integrate artificial intelligence for better automation. |
| Security - 10 | Provides role-based identification, log-in, and access control for each module; offers PDF printing capability; tracks document changes with detailed audit trails, as required by the FDA; electronic signatures with timestamps for document approval. |
| Value - 7 | Starts at $799 per month, which includes implementation fees; offers a 30-day free trial; pricing structure can be confusing, tacking on separate fees for basic users and backup and maintenance support. |
| Performance - 10 | Offers fast, responsive interface; no wait time when navigating between modules. |
| Key Features - 10 | Generates customizable reports for monthly, quarterly, and annual performance metrics; automates audit trails and notifications for past due calibrations and document modifications; allows customization of documents based on classifications; includes analytics capabilities that enable users to analyze data and trends within their quality management processes. |
Who Uses Qualcy eQMS?
We found Qualcy well-suited for organizations with 5 to 500 users, but it can support up to 1,000. It’s the next logical step for users transitioning from manual or semi-digital methods to a cloud-based system. The QMS operates best in the medical device, pharmaceutical, and biomedical sectors. Qualcy offers on-premises and cloud-based deployment secured and backed up on the Amazon AWS platform.
What Features Are Missing?
- Simple setup: Qualcy Systems works closely with companies to implement their QMS system. However, some customer reviews note that the initial setup process can be extensive, particularly when configuring user permissions and access controls. Additionally, learning to work with risk management templates and setting up the document management section consumes considerable time.
- In-app guidance: On the usability front, users have expressed that, at times, the functionality of specific sections within modules is not immediately clear. This is compounded by the absence of a general help menu with quick answers to queries about the software’s basic mechanics.
Pricing Plans
Qualcy operates on a monthly subscription model, where the fee is based on the number of admin users. Admin users are granted full access and control within the system. In contrast, basic users have restricted permissions and are priced differently, with fees ranging between $21 and $39 per month, depending on the total number of users in the account.
For most companies, pricing starts at around $399/month. It’s $99/admin user plus the $299 monthly backup and maintenance support fee.
| Plan | Pricing | Features |
| Basic | $99/user/month + $299/month backup and maintenance support |
|
| Gold | $129/user/month + $399/month backup and maintenance support | Includes Basic Features and:
|
| Platinum | $149/user/month + $449/month backup and maintenance support | Includes Gold features and:
|
Who uses Qualcy eQMS?
Qualcy customers include biotech companies like Activa Biotech, Bioquest, Inc., Bioserv Corporation, Innovation Cell Technologies, and Pacific Biosystems Inc. The QMS software is best suited for small to medium-sized enterprises that must comply with specific regulations like ISO. Qualcy eQMS also helps ensure compliance with FDA regulations such as 21 CFR Part 11, which includes requirements like audit trails, electronic signatures, and unique user IDs.
How is eQMS different from QMS?
QMS is a broad term referring to the procedures and policies utilized by an organization to meet product and service quality standards. It can take the form of documented manuals and paper-based records. eQMS refers to a digitized system managed through software, making it more efficient and compliant with regulatory requirements.
Alternatives
Summary
In our evaluation, Qualcy eQMS emerged as an adaptable solution catering primarily to medical device manufacturers, engineering firms, and professionals within the biomedical industry. Prospective users can gauge the software’s efficacy through a 30-day free trial. Furthermore, the platform offers flexibility, allowing customers to use individual modules that cater to specific needs or opt for the comprehensive system. Moreover, the platform takes data security seriously, employing high-grade safeguards to protect sensitive information.
We recommend potential users weigh Qualcy’s extensive features against the initial setup time and potential learning curve. Companies must select the Platinum subscription tier for advanced implementation and data migration support. Additionally, organizations should prepare to allocate time to devise initial training schedules, configure user roles, and establish risk management protocols.
In our assessment, we think Qualcy is a great fit for small to mid-level biomedical companies looking to tighten their security and maintain ISO 13485 standards. Its modular nature, user-friendly interface, and strong features make it an asset for organizations keen on optimizing their quality management efforts.
User Reviews of Qualcy eQMS
Write a Review- Biotech
The Qualcy team provides outstanding customer support
Although I had prior experience with TrackWise, another QMS platform, that familiarity did not translate to using Qualcy. I found the system’s processes less intuitive without direct guidance from our Qualcy support contact. As a new user working to help our small business understand and implement a compliant QMS, I’ve primarily used Qualcy to upload, review, revise, and approve documents. I have not yet explored the collaboration, training, or competency modules. The document control functionality has worked very well for our needs, and we are pleased with this aspect of the program. I appreciate the flexibility the system offers in using Excel to build and upload templates. Once I understood how to format the templates and saw the output, I was very satisfied. The resulting forms looked professional and clean.
Pros
The Qualcy team provides outstanding customer support. They are consistently responsive, thorough, and patient. Whenever I encounter a challenge within the system, I feel fully supported—with timely responses and the option to work through solutions via Zoom meetings. Sanjay, in particular, has been instrumental in getting me back on track quickly and effectively.
Cons
I would like to see more training videos provided for key functions as most are not intuitive to navigate.
Qualcy eQMS Review
The service is fantastic. Our POC is very responsive, knowledgeable and patient. Their attention to detail and effective training make our eQMS efforts both enjoyable and successful.
Pros
We really like the ability to do traceability between requirements docs within the eQMS. And the system is very comprehensive.
Cons
In many environments, the day to day reality of "doing quality" can be challenging. It's more of a behind the scenes function, unlike more "glossy" departments like sales, engineering or executive management. Having integration with traceability forces the quality function to interact with engineering functions more frequently, which is good for efficiency, but some engineering personalities can be challenging.

- Health Care Providers & Services
Qualcy is great for doc control, equiptment management
Qualcy is great for doc control, equiptment management (including calibration and PM reminders), audit, CAPA, and NC management. it is slim, so a great fit for a company that moves quickly.
Pros
Qualcy is a surprisingly customizable tool for eQMS management. the support team is very responsive when you have questions and the tool can support paperless quality records without a huge effort to develop the system.
Cons
there is not an obvious support path other than contacting the qualcy team. ideally there would be more documentation on each module.
- Internet Software & Services
- 1-10 employees
- Annual revenue $0-$1M
The simplicity and user interface is a big plus
Qualcy eQMS is user friendly, easy to find the documents and records. The change control feature has been very helpful for us. The Training Management module is integrated with change control, this automatically releases the training assignments, when documents are changed. This has been a big difference, previously we used to spend lot of time on managing the training records. The other modules including CAPA, NC Management and Risk Management are integrated, which makes it easier to link the documents and QMS records.
Pros
The simplicity and user interface is a big plus. The advanced notifications for tasks and approvals has been useful. The Qualcy team provides excellent support, we are surprised to find that they have team members who have GMP experience, that makes a big difference. They understand the issues and provide feedbacks that are aligned with our GMP/QSR expectations.
Cons
The software comes with nice reports, but they are not easy to drill down. Though same data can be obtained through Excel downloads. It takes more steps to get the same data.
