What is FSMA 204? A Complete Guide to Food Traceability Compliance
Learn how the FDA’s Food Traceability Rule impacts growers, manufacturers, distributors, and retailers.
The U.S. Food Safety Modernization Act (FSMA) Section 204, also known as the Food Traceability Rule, establishes a nationwide standard for how food companies must document, store, and share supply chain data on certain high-risk foods. The rule requires improved recordkeeping for items on the Food Traceability List (FTL). It demands that records be made available within 24 hours upon request of the U.S. Food and Drug Administration (FDA).
While the formal compliance date has been extended to July 20th 2028, the market is already adapting. Food manufacturers, processors, and distributors must begin aligning their operations now to avoid disruptions and potential contract losses. Many are already modernizing with tools like food manufacturing and food distribution software that digitize traceability automatically.
In this guide, you’ll learn:
- What the Food Traceability Rule covers and who must comply
- The requirements around traceability events and key data elements
- Compliance deadlines and what they mean for your business
- How can different roles in the supply chain prepare now
- The tools and software available to help you meet these requirements
Understanding the Food Traceability Rule
FSMA 204 builds on the broader Food Safety Modernization Act, designed to shift the U.S. Food system from a reactive to a proactive approach. Section 204 focuses specifically on traceability, or the ability to follow food through every step of the supply chain—from harvest to final sale.
By doing so, the FDA aims to reduce the number of foodborne illnesses by improving recall efficiency to remove at-risk foods from shelves quickly.
Foods covered under the FTL
The rule applies to only the foods listed on the FDA’s Food Traceability List. These items are identified as higher risk for causing foodborne illnesses or contamination. Many of these foods made the list due to previous recalls or food safety incidents.
A few categories include:
- Fresh produce such as leafy greens, melons, herbs, and tomatoes
- Soft and semi-soft cheeses
- Shell eggs
- Nut butters
- Seafood and crustaceans
- Ready-to-eat deli salads and other refrigerated items
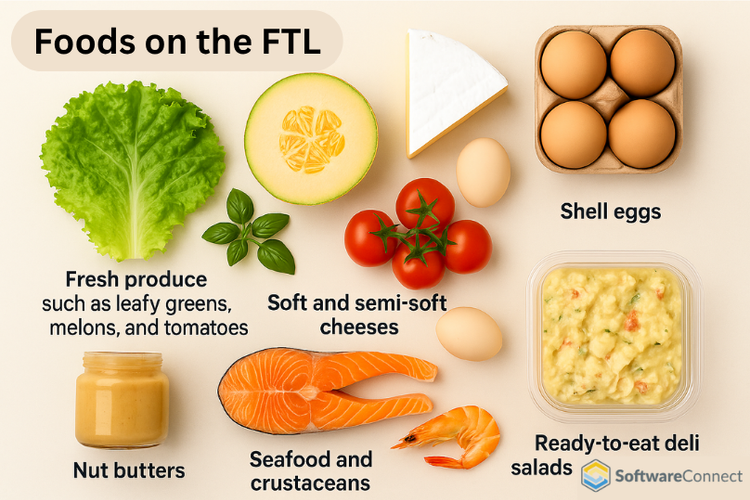
The complete FTL product list is available for download on the FDA website here.
Businesses dealing with these foods (or foods that contain these items as ingredients) must determine if they’re subject to the rule and then establish traceability systems accordingly.
Risks of non-compliance with FSMA 204
Failing to meet the FSMA 204 standards provided by the FDA can lead to businesses receiving FDA warning letters or mandatory recalls. Continued failure to comply with FSMA 204 may result in civil penalties under the Federal Food, Drug, and Cosmetic Act. However, at this time, the FDA has not announced any details regarding the fines or penalties that businesses may face.
The current risk of noncompliance is losing supplier status with major customers like Walmart and Albertsons.
FSMA 204 Requirements and Compliance Checklist
FSMA 204 establishes clear expectations for capturing and maintaining traceability data throughout the food supply chain. The rule goes beyond basic recordkeeping and requires companies that manufacture, process, pack, or hold foods on the FTL to document each step of the supply chain.
To meet these obligations, businesses must:
- Assign and retain a Traceability Lot Code (TLC) for every food item or product
- Record Key Data Elements (KDEs) during defined Critical Tracking Events (CTEs)
- Maintain a written traceability plan describing how data is captured, stored, and shared
- Retain all records in an electronic, sortable format that can be provided to the FDA within 24 hours of request
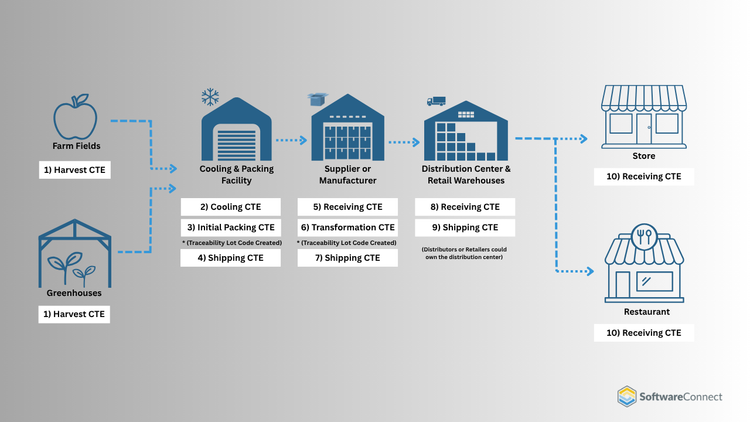
What are Critical Tracking Events (CTEs)
CTEs are events that occur anytime a food item is moved or changes form. According to the FDA guidelines, these are the seven critical events required for food items on the FTL:
- Harvesting
- First land-based receiving (for seafood items)
- Cooling (before initial packing)
- Initial packing of raw items
- Shipping
- Receiving
- Transformation (repacking, processing, manufacturing events)
What are Key Data Elements (KDEs)
For each CTE your business performs, FSMA 204 mandates that specific data be recorded at each event. For example, at the harvesting event, you must capture:
- The product’s description, quantity, and unit of measure
- The harvest date and field (for produce) or landing location (for seafood)
- TLC assigned by the harvesting entity or next handler
- Reference document type and number (bill of lading or invoice)
Similarly, for shipping events, you must record:
- TLC for the food item
- Product description, quantity, and unit of measure
- Date of shipment, location of the sender, and location of the recipient
- Reference document type and number (bill of lading or invoice)
Traceability Plan
Every business covered by FSMA 204 must retain a copy of a written traceability plan. This plan should describe:
- How foods on the FTL are identified and tracked
- The procedures for assigning lot codes and capturing KDEs
- The format, location, and retention period for digital records
- A point of contact for all traceability questions and requests
These records must be maintained and current for at least two years. If you update or reform your traceability plan, you must also retain the prior version for at least two years.
FSMA 204 Compliance Dates and Deadlines
The Food Safety Modernization Act, Final Traceability Rule (FSMA 204), formerly titled “Requirements for Additional Traceability Records for Certain Foods,” was issued on November 21, 2022. The rule originally required covered entities to comply by January 20, 2026.
However, on March 20, 2025, the FDA announced its intention to delay enforcement by 30 months, moving the new enforcement date to July 20, 2028. The agency cited feedback from food industry leaders who expressed concern about their trading partners’ readiness, noting that many of their suppliers still lacked the necessary infrastructure to meet the new digital standards.
While the extension grants additional time, it does not change the requirements of FSMA 204. Companies will still be expected to maintain electronically sortable traceability records for items on the FTL list. Because compliance relies on consistent data sharing across all supply-chain partners, the extension should be used as an opportunity to test systems and confirm partner alignment, rather than delay preparation.
Many large retailers and food buyers, including Walmart and Albertsons, are already incorporating FSMA 204 traceability clauses into their supplier contracts, effective August 1, 2025. Food companies that act early will be better prepared to retain vendor relationships and avoid potential disruptions once enforcement begins.
How Does FSMA 204 Affect Businesses?
FSMA 204 impacts nearly every organization that handles or processes food items on the FDA’s Food Traceability List. However, the obligations will vary depending on your role in the food supply chain. Understanding your verticals’ requirements and responsibilities will help guide your compliance strategy.
Growers, Packers, and Fresh Produce
Growers, packers, and produce suppliers sit at the foundation of the traceability process. Their responsibility begins at the initial harvest, when food is first removed from its source. They must capture harvest dates, field or plot identifiers, and the quantity of produce collected. They need to carry that data through the cooling and initial packaging of the product.
A traceability lot code is assigned during this stage, linking all future records back to the point of origin. For growers supplying multiple buyers or working through cooperatives, standardizing lot codes and data formats prevents traceability issues later in the supply chain.
Seafood and Aquaculture
Seafood operations follow a similar process, but begin their record-keeping when the food item leaves the fishing vessel and reaches the dock for the first time. Once the item reaches land, these businesses must document fishing vessel identifiers, catch areas, and landing dates. They must also carry these details as the seafood moves through processing and finally to cold storage, where the first traceability lot code is created.
Temperature control and product transformation are common for this vertical, making electronic systems critical for maintaining visibility and compliance from initial catch to final sale.
Manufacturing and Food Processing
Once products enter manufacturing and processing, the focus shifts to what FSMA 204 defines as a product “transformation.” This occurs whenever ingredients are repacked, blended, or converted into a new product. Manufacturers must assign new traceability lot codes while maintaining a link to all source ingredients and their respective TLC. They must document the facility location, production date, and reference documents for every batch.
Because manufacturers handle large amounts of raw materials for every transformation, keeping track of origin TLCs can be problematic without traceability software. The result is a digital record that ties finished goods back to their raw inputs, something that paper records just can’t handle.
Distributors
Distributors and wholesalers connect the entire supply chain. Their traceability responsibilities are centered around shipping and receiving events. Whenever they handle a product, they must ensure that data such as lot codes, product descriptions, and quantities remain intact throughout every movement. Distributors must also record the original source of the products and the destination to which they will be shipped.
Because they handle high volumes across multiple suppliers and retailers, creating a consistent process is key. Many distributors are now implementing GS1-compliant labeling or electronic data interchange (EDI) systems that make the sharing of KDEs automatic and standardized.
Retailers and Grocers
For food retailers, FSMA 204 is less about capturing the data firsthand and more about proving that their suppliers are compliant. For every product that they sell on the food traceability list, they must be able to trace it back to its source and store the supplier-provided details in an electronic, sortable format. This ensures that in the event of a recall, they can quickly identify the affected products and demonstrate their FSMA compliance to FDA regulators.
Retailers that operate private-label food programs face the additional responsibility of ensuring their co-packers and third-party distributors meet all FDA requirements.
Importers
Although based abroad, foreign suppliers share the same accountability as domestic distributors. Foods covered by FSMA 204 must arrive in the U.S. with full traceability data that aligns with the established KDE and CTE definitions. Importers are responsible for verifying that their foreign trading partners can provide these records within 24 hours of an FDA request.
Many importers seek import and export software to help them stay organized and in control of their product data. Import and export software also assists with customs and transportation management.
Software for FSMA 204 Compliance
Meeting FSMA 204 requirements is nearly impossible without the right technology. The food traceability rule mandates that all records must be stored in an electronic, sortable format, accessible within 24 hours of an FDA request. For many businesses still relying on paper logs, spreadsheets, or legacy ERP systems, this means it’s time to invest in software designed for modern traceability.
Why Software Matters for FSMA 204
Digital traceability systems ensure Key Data Elements and Critical Tracking Events are captured, stored, and shared according to FSMA 204 requirements. Instead of manually updating spreadsheets, these platforms automatically capture product data consistently, so traceability lot codes remain intact throughout the supply chain.
Software also makes compliance audits faster and less stressful for businesses. When records are centralized and searchable, you can produce a complete product history in minutes, meeting FSMA 204’s 24-hour requirement.
Core Features to Look For
When evaluating FSMA 204 compliance software, you should look for tools that provide:
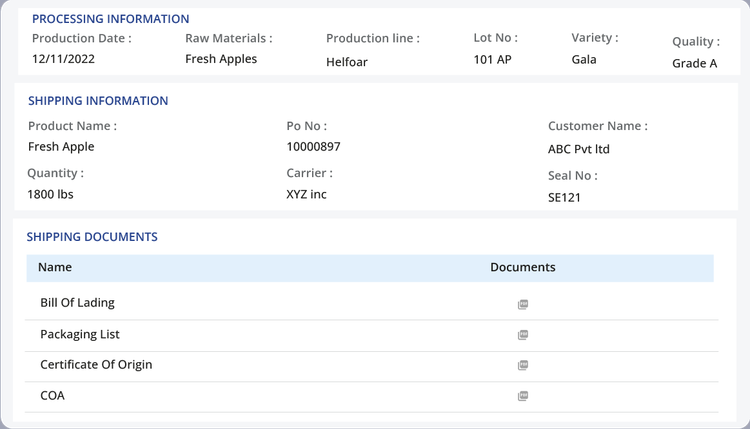
- Digital data and document management: Digitally store all KDEs, traceability records, and relevant documents (BOLs & invoices) in one secure database.
- Automated lot-code assignment: Generate and link traceability lot codes to food products and critical events automatically.
- Barcode and GS1 data capture: Use standardized labels to reduce manual data entry errors and ensure interoperability with trading partners.
- Audit-ready reporting: Create required reports quickly and share traceability data with FDA regulators and trading partners.
- Integration with existing systems: Connect traceability systems to current ERP, WMS, or EDI platforms to ensure all related product data is organized and consolidated.
Choosing the Right System
Choosing the right software depends on your operation size, product offerings, and supply-chain complexity. Decisions also depend on what your existing software can handle and what data it captures automatically.
Some organizations implement specialized traceability platforms focused solely on FSMA 204 data capture and lot tracking. In contrast, others select all-in-one solutions, like an ERP or warehouse management software which combine inventory, production, and compliance management in one system.
To learn more about these systems, visit our food traceability, food distribution, or food manufacturing pages built for your specific industry.
Next Steps
If your traceability process still relies on manual data entry or disconnected spreadsheets, now is the time to upgrade. The FDA is using FSMA 204 as a regulatory means to modernize the U.S. supply chain and ensure food and beverage companies are accountable.
