FactoryTalk PharmaSuite
1 Review 2/5 ★ ★ ★ ★ ★A manufacturing execution system developed for the life sciences industry featuring master recipe maintenance and GMP life cycle management.
Product Overview
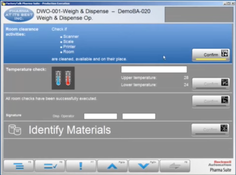
Rockwell’s PharmaSuite is a manufacturing execution system designed for the life sciences industry. Key features include production equipment management, intermediate faults tracking, and real-time quality dashboards. PharmaSuite delivers role-based optimization for each lifecycle stage. It also provides a live view of the shop floor and integrates with ERP systems as well as the automation layer. PharmaSuite’s compatibility with ISA S88 standards ensures enhanced process consistency and quality control.Pros
- Real-time reporting for improved production control
- Integrates with ERP, warehouse management, and laboratory info systems
- Features compliant recipe design functions
Cons
- Requires significant on-premise infrastructure
- Complex setup
Target Market
Mid-sized to large pharmaceutical and biotech companies seeking to modernize their manufacturing processes, focusing on compliance, data integrity, and efficiency.PharmaSuite from Rockwell Software is an advanced MES designed for the pharmaceutical industry. It provides an integrated solution to manage and optimize production processes, ensuring compliance with industry standards and regulations. PharmaSuite streamlines manufacturing operations, enhances production efficiency, and ensures product quality and consistency.
PharmaSuite Key Features:
- Goods Manufacturing Practice (GMP) Life Cycle Management: Offers comprehensive management of the GMP life cycle, ensuring that products are consistently produced and controlled according to quality standards.
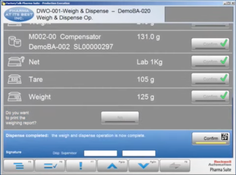
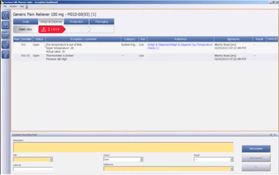
- Master Recipe Maintenance: Facilitates the creation, management, and storage of master recipes, providing a centralized system for recipe information and revision control.
- 21 CFR Part 11 Regulatory Compliance: Ensures compliance with the FDA’s 21 CFR Part 11 regulation, which sets criteria for electronic records and electronic signatures, vital for the pharmaceutical industry.
- Batch Record Generation: Automates the generation of batch records, improving accuracy and efficiency while reducing the potential for human error.
- Integration with ERP Systems: Seamlessly integrates with Enterprise Resource Planning (ERP) systems, facilitating better coordination between manufacturing and business operations.
PharamSuite Benefits:
- Enhanced Compliance: Assists in meeting regulatory requirements, reducing the risk of non-compliance and associated costs.
- Increased Efficiency: Streamlines manufacturing processes, reducing waste and increasing throughput.
- Improved Product Quality: Ensures consistent product quality, enhancing customer satisfaction and trust.
Product Overview
Developer Overview
Related Products
User Reviews of PharmaSuite
Write a ReviewOne of the most difficult to deploy MES/MOM systems
One of the most difficult to deploy MES/MOM systems with weak and expensive EU support.
Pros
Can be a robust system, if once you are able to deploy the elements of system you need.
Cons
Still no available validate cloud capable version released. RA has acquired Plex MES aiming to have this option.