IQS Quality Management
2 Reviews 5/5 ★ ★ ★ ★ ★- Acquired
- Discontinued
IQS was a quality management software that managed complex compliance requirements across various industries. It was acquired in 2017. Its features were migrated to Cority Quality Cloud. We recommend looking at our quality management software roundup for alternatives, or talk to one of our software advisors for help.
Product Overview
IQS was a quality and compliance management software system acquired by Cority in 2017. It featured process review tools, compliance reporting, and policy drafting capabilities. It was also available in on-premise and cloud-based formats. Overall, it served to optimize risk reduction and cost effiency across multiple industries.Pros
- Extensive help resources for questions and potential customizations
- Modular architecture was adaptable to specific company needs
- Compliant with ISO 9001, IATF 16949, etc.
Cons
- Unintuitive user interface
- No self-reset option for passwords
- Acquired in 2017
Target Market
Small to enterprise-level companies with anywhere from 50 to over 1,000 employees. Suitable for the automotive, medical device, aerospace, and defense industries.IQS was integrated into Cority’s suite of products, now operating under the umbrella of Cority Quality Cloud. This integration occurred following Cority’s acquisition of IQS in 2017.
IQS Alternatives
1 Waypoint Global Suite
A manufacturing-focused solution for document management and quality control, offering process review tools, compliance reporting, and policy drafting capabilities, available in both on-premise and cloud-based formats.
2 TrackWise
A cloud-based enterprise quality management solution optimizing quality, compliance, risk reduction, and cost efficiency across various industries.
3 EtQ Reliance
A cloud-based quality management system that can be tailored to various industries, from life sciences to food and beverage, with over 40 applications.
IQS Key Features
- Audit, change, and complaint management
- Document and employee training
- Equipment maintenance
- FMEA/control plans
- Gauge calibration
- Inspections/SPC
- Nonconformance/CAPA
- PPAP/first article inspections
- Supplier performance
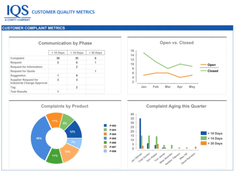
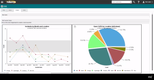
- Analytics emphasized the growing importance of business analytics tools, particularly in the manufacturing sector, for data-driven decision-making.
IQS business analytics offered advantages like true business intelligence, ad hoc reporting, and real-time data-driven decisions. The software provided graphical data representation, multiple format conversions, and accessibility across various platforms. Installation and usage were straightforward, enabling efficient data visualization, mining, analysis, and integration.
Audit management Software from IQS addressed the challenges of audit coordination, focusing on internal, third-party, and supplier audits, integrating audit documentation, and facilitating feedback from internal audits.
Change management software emphasized the need for rapid adaptation to product design changes, highlighting the importance of communicating alterations efficiently.
Document management software addressed regulatory compliance challenges, offering an integrated application for revision control protocols and streamlining the change approval process.
Employee training software provided a unified interface for training initiatives, assessing employee skills, and managing training records.
Equipment maintenance software focused on ISO 9000 standards, offering automated preventive maintenance management and documentation.
FMEA/control plan/process flow software was crucial for evaluating design process elements, particularly in industries with stringent regulatory mandates.
Gage calibration software ensured process quality data integrity, automating MSA device analysis and calibration schedules.
Inspection/SPC software offered solutions for inspection challenges and statistical process control, integrating inspection results with other IQS modules.
Nonconformance/CAPA software optimized NCM procedures and corrective and preventative actions, integrating disparate software systems into a unified solution for efficiency.
Product Overview
Developer Overview
Related Products
User Reviews of IQS
Write a Review- Food Products
- 50K-100K employees
- Annual revenue $10B+
IQS Review
This helps to record the entire data.
Pros
This IQS helps to implement the SPC on shop floor which helps to stabilize the process.
Cons
Nothing. When we go deeper into it, will get more thoughts.
It's a software that runs our company
The software does a really good job of meeting our needs in a lot of different areas from human resources to our maintenance department and of course our quality department. Everybody in our business uses IQS. It really is at the heart of our company now. It’s a software that runs our company and has really changed how we do business today.