The Best Pharmaceutical Manufacturing Software
Pharmaceutical manufacturing software manages the complex processes involved in drug production while maintaining compliance with the FDA. We evaluated various systems based on factors like quality control testing and expiration date management.
- Comprehensive formula and recipe management
- Seamless integration with leading financial systems
- Includes quality control and compliance features
- Enhanced regulatory compliance
- Optimized supply chain management
- Offers tailored features like recipe and lab management
- Recalls and mock recalls are easy to perform
- Greatly increases the ability to fill orders
- Wide variety of reports
Using our advanced review methodology, we’ve reviewed the best solutions for companies of all sizes, from small pharma companies to biomed enterprises, and listed our favorites below.
- BatchMaster ERP: Best Quality Control Testing
- Datacor ERP: Best Expiration Date Tracking
- Mar-Kov: Best Production Cost Analysis
- Aptean Process Manufacturing: Best Formulation and Recipe Management
- Acctivate Inventory Management: Best for Nutraceuticals
- QT9 ERP: Strong Regulatory Compliance
- Deacom: Strong Batch Tracking
- QAD ERP O³: Best for Enterprises
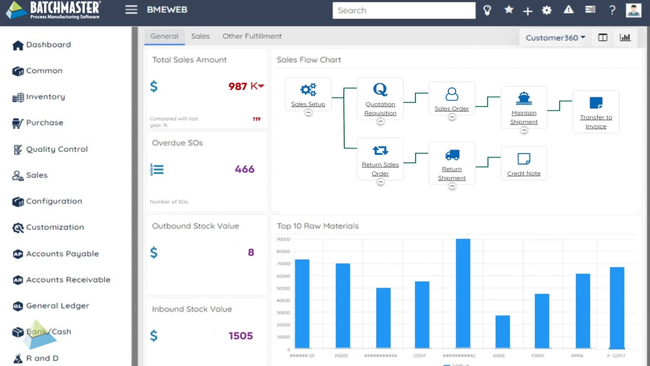
BatchMaster ERP - Best Quality Control Testing
BatchMaster’s quality control testing capability is designed to ensure the highest product quality standards and compliance in the manufacturing process. The system enables you to create and execute inspections at every stage of the production process, including raw material, in-process, and finished goods testing.
For example, maybe you want to test a batch of antibiotic tablets. During production, BatchMaster ERP prompts checks for uniformity of dosage units and dissolution rates. If the system detects any deviations, production will stop, and the batch will be put into quarantine and flagged for further analysis.
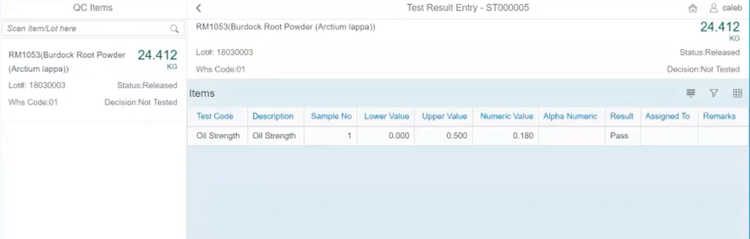
Before packaging the tablets, they’ll undergo stability testing and microbiological analysis. The system logs all results, and if the parameters meet the standards, the batch is approved for release. BatchMaster will generate a compliance report with audit trails to ensure you meet FDA requirements, such as Good Laboratory Practices (GLPs) and Current Good Manufacturing Practices (cGMPs).
View our product profile on BatchMaster ERP for more details.
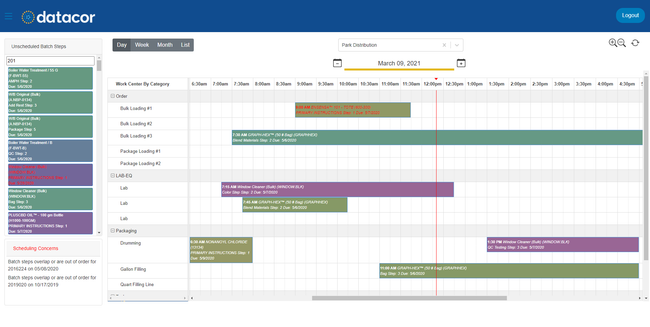
Datacor ERP - Best Expiration Date Tracking
Datacor ERP’s shelf-life tracking feature enables you to manage stock based on expiration, ensuring that you use prescriptions before they go to waste. The system automatically assigns the dates when products enter inventory through production, purchase orders, or warehouse receipts.
For example, let’s say you’re producing insulin vials. Using Datacor ERP, you can assign this batch a shelf life of four months. The system will automatically track all expiration dates and make them accessible across all departments.
Over time, your system alerts you that 15% of your batch will expire in six weeks. Leveraging the FEFO picking method, your warehouse team can prioritize the near-expiring products for upcoming shipments. This capability supports compliance with regulatory standards set by the FDA and can help minimize prescription waste.
However, Datacor’s pricing starts at $1,500 monthly, which can be costly for smaller manufacturers. If this is the case, you might want to consider a more affordable solution, such as Deacom Essentials starting at $500 per month, or Mar-Kov at $159/month.
For more information, read our Datacor ERP product review.
Mar-Kov - Best Production Cost Analysis
Mar-Kov enables accurate cost calculation by considering factors like raw materials, labor, overheads, equipment usage, and packaging. With these precise insights into your production, you can optimize your operations and maximize profitability.
Mar-Kov integrates with inventory systems to pull real-time data on material costs and stock levels and syncs with production schedules to track resource usage. The software continuously updates cost calculations as new data arrives, ensuring accurate and timely data.
If you produce a 500-unit batch of pain relievers, you’ll likely source your active ingredients from multiple suppliers. Using Mar-Kov, you can calculate the total cost of the batch, including API costs, excipients, labor, and overhead, which comes out to $9,500, or $19/unit.
During production, you discover that some of the materials are spoiled, quantifying the waste cost at $300. Based on the actual batch cost of $19/unit, the system suggests a minimum selling price of $30/unit to ensure a healthy margin and stay competitive. The software will also track additional costs associated with quality control testing and certification to meet FDA compliance.
Mar-Kov’s pricing starts at $321/month, making it our most affordable product pick. This makes it accessible for smaller manufacturing companies in the fragrance and cosmetic sectors.
View our full Mar-Kov product review for more information.
Aptean Process Manufacturing - Best Formulation and Recipe Management
Aptean Process Manufacturing ERP allows you to create, modify, and store recipes and formulations in the database, ensuring consistency across all production facilities.
For example, maybe you’re developing a new pain relief gel for athletes. First, you create the initial formulation, specifying your active ingredients, like menthol, excipients, and thickening agents. You can enter these details into the database with ingredient specifications and concentration ranges. Multiple iterations of the formulation will be developed and tested in the lab. You can track each version in the system, along with results from lab tests like stability, efficacy, and viscosity.
Before production, the system checks the inventory for required raw materials. If there is a delay in the supply chain and substitutions are needed, it will suggest compliant alternatives.
Aptean will analyze the cost of each iteration, helping you choose an effective and cost-efficient recipe. When you have a final recipe, quality assurance and regulatory teams will review the formulation to ensure compliance with FDA guidelines for labeling and safety.
Once approved, you can scale it up for mass production. The software will automatically adjust the ingredient quantities based on the batch size. Aptean can also flag potential allergens, ensuring proper labeling for consumer safety and compliance.
However, some users reported delays in getting timely support or resolution to their issues with the software, which can hinder productivity.
To discover more details about Aptean, read our full product review.
Acctivate Inventory Management - Best for Nutraceuticals
Acctivate Inventory Management’s batch process control with end-to-end traceability makes it a great choice for neutraceutical manufacturers. The system supports an unlimited number of products through inventory, assigning lot or serial numbers to each. It even lets you have lot numbers for ingredients used, allowing for more control and stronger traceability.
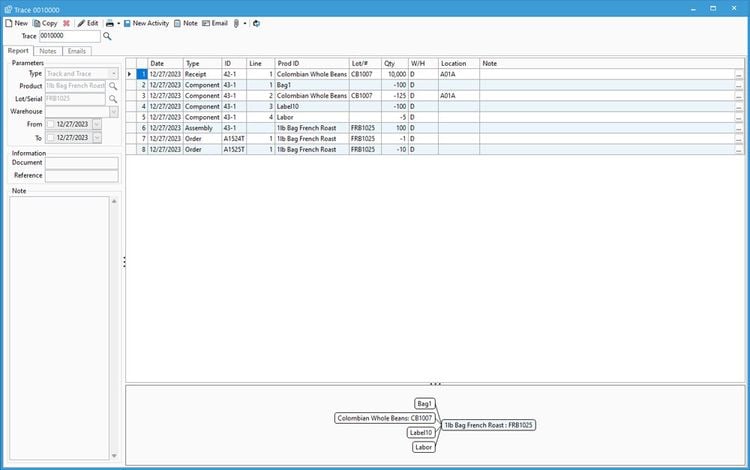
Additionally, the system can help you manage batch production outputs. You can add recipe variations to allow for substitute items or quantity changes to stay flexible. Also, you can track yield variance for every lot, ensuring consistent output quality. This is essential for nutraceuticals to meet label claim accuracy and comply with 21 CFR Part 111, which requires accurate batch records.
Acctivate is best for companies currently using QuickBooks, as it seamlessly integrates with that system for accounting. It’s ideal for small to midsize companies that don’t want to make the jump to a full ERP system. This is reflected in the affordable price, with the multi-user Professional plan starting at $8,000/year.
See our full Acctivate Inventory Management review.
QT9 ERP - Strong Regulatory Compliance
The pharmaceutical industry is highly regulated, and non-compliance often results in hefty fines, product recalls, and, in extreme cases, shutdowns. QT9 ERP helps ensure adherence to standards set by the FDA, EMA, and other governing bodies. This capability integrates quality management and compliance tracking into the ERP system, enabling you to maintain audit readiness.
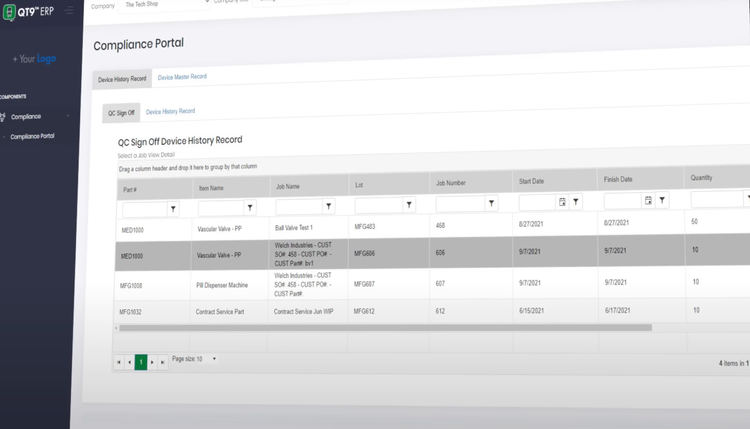
The software offers centralized storage and control of Standard Operating Procedures (SOPs), policies, and compliance documents. It adheres to FDA 21 CFR Part 210 and Part 211, including FDA 21 CFR Part 11 electronic signatures. The intuitive platform includes one-click master batch records and electronic batch records capabilities, with barcoding integrated to ensure accurate material tracking and full traceability throughout production.
QT9 allows you to better prepare for an FDA inspection. The database includes a document control module that ensures that all SOPs and work instructions are up-to-date and approved. All your employee training will be assigned and tracked within the system.
Using the audit management tool, you can schedule a mock FDA audit, where the software will provide you with a checklist based on FDA guidelines to simulate an inspection. When inspectors request batch records, you can easily retrieve them electronically, showing end-to-end traceability for raw materials, production, and quality testing. The audit trail functionality will highlight all changes made to essential documents.
QT9 may lack the scalability required for large enterprises with complex operations. While it excels in regulatory compliance management, its functionality might not be as comprehensive as more enterprise-focused solutions.
Read our QT9 ERP review for more details.
Deacom - Strong Batch Tracking
Deacom’s batch tracking feature ensures complete traceability and compliance. The system enables you to manage raw materials, work-in-process items, and finished goods from production to distribution.
Once you receive APIs and assign lot numbers, Deacom will inspect the materials and log their lot details. Operators can follow the predefined workflows stored in the electronic batch records during production. You can track each stage in real-time, from mixing to tableting and coating. The software automatically flags the batches for inspection if a machine’s output doesn’t meet predefined specifications.
Before final packaging, the system triggers quality-control tests and records results directly in the electronic batch record, ensuring that every product meets quality standards. All completed items are assigned batch numbers that trace back to the raw materials and production processes. If you receive a quality issue for one of these items, you can use Deacom to quickly trace the affected batches, identify the root cause, and recall products efficiently. Deacom Essentials’ pricing starts at $500 for the Essentials plan but can increase to $5,000 for the full ERP.
Visit our Deacom product page for more details.
QAD ERP O³ - Best for Enterprises
QAD’s production planning and scheduling enable you to create a master schedule that aligns activities with demand forecasts.
For instance, maybe you specialize in oncology drugs and are facing an increased demand for a newly approved medication. Ideally, you need to ramp up production fast while maintaining compliance with FDA regulations.
QAD identifies underutilized production lines and allocates them for the new drug while ensuring that other products in the portfolio are not delayed. The software calculates the exact amount of APIs and excipients required for the increased production and issues purchase orders to suppliers, ensuring on-time delivery.
If an unexpected delay occurs, such as a machine breaking, QAD will automatically reschedule batches to alternative lines, prioritizing the oncology drug without disrupting ongoing production. The database ensures each batch adheres to the required cleaning validation protocols before switching to different formulations, minimizing contamination risks. The system will track and log all regulatory documentation as batches are produced, ensuring readiness for FDA inspections and audits.
QAD is heavily focused on compliance and quality management, which could be overly complex for smaller companies. Ideally, this product is best suited for large pharmaceutical manufacturers.
Other Systems We Recommend
Sage X3 is another good option for pharmaceutical and biomed manufacturers. This ERP system offers strong quality control and supply chain traceability tools that allow midsize businesses to gain more control over production and ensure compliance with regulations.
Systems We Don’t Recommend
NetSuite is not natively built to support pharmaceutical manufacturing. You will need to purchase additional third-party add-ons like blendAPPS to make it a viable option. Still, at that point, you’re better off purchasing a system with native pharmaceutical functionality.
What is Pharmaceutical Manufacturing Software?
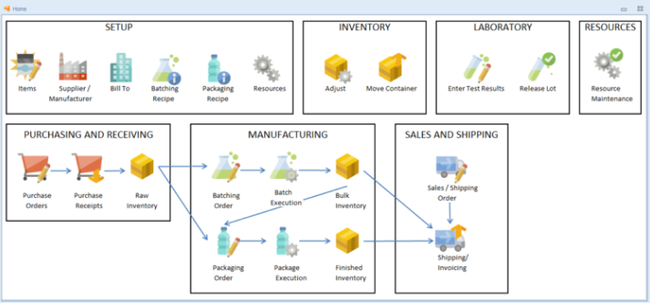
Pharmaceutical manufacturing software is designed to support pharmaceutical production’s unique processes and compliance requirements. These systems can help pharma and biomed manufacturers and production teams streamline operations, meet FDA-compliance standards, and maintain product quality.
Pharmaceutical ERP software provides process manufacturing functionalities such as recipe management, batch tracking, expiration management, quality control, compliance tracking, and production scheduling. The software also provides features found in most manufacturing or material resource planning (MRP) options, including improved inventory control, order management, proper purchasing, and general accounting.
Common Challenges
- Stringent Regulatory Compliance: Manufacturers must adhere to strict regulations from the FDA and EMA. Systems with automated monitoring and audit trails can help you remain compliant.
- Quality Control and Assurance: Ensuring consistent product quality while managing raw materials and formulations is critical to avoid costly recalls. A system with quality control testing and real-time monitoring can analyze raw materials and track production parameters to identify deviations early.
- Cost Management: High compliance costs and supply chain inefficiencies can disrupt your profit margins. A system that tracks expenses at every stage and optimizes inventory can prevent overstocking and stockouts and help you stay on track.
- Production Delays: Balancing fluctuating demand, limited resources, and complex processes can lead to production delays. Planning tools can optimize schedules based on available resources, demand, and deadlines.
Key Features
- Electronic Batch Records (EBR): Tracks and stores detailed records of all manufactured batches to ensure compliance with FDA’s 21 CFR Part 11.
- Quality Control and Assurance: Monitors product quality throughout manufacturing and integrates testing protocols for raw materials, in-process products, and finished goods.
- Compliance Management: Ensures adherence to global regulations such as FDA, GMP, and ISO standards.
- Recipe and Formula Management: Stores and manages recipes for various pharmaceutical products and ensures accurate ingredient proportions for different batch sizes.
- Production Planning and Scheduling: Optimizes production schedules based on resource availability and demand forecasts.
- Materials Management: Tracks raw materials, in-process inventory, and finished goods in real time. It also manages expiration dates, shelf-life, and reorder points.
- Costing and Profitability Analysis: Tracks production expenses at each manufacturing stage and provides cost breakdowns for raw materials, labor, and overhead.
- Supply Chain Management: Manages supplier relationships, contracts, and performance, providing visibility into raw material availability and tracking shipment.
- Labeling: Ensures proper labeling of products, including barcodes, batch numbers, and expiration dates.
Pricing Guide
| Software | Best For | Deployment Options | Starting Price |
|---|---|---|---|
| BatchMaster ERP | Quality Control Testing | Cloud or On-Premise | $2,000/user/one-time |
| Datacor ERP | Inventory Management | Cloud or On-Premise | $1,500/month |
| Mar-Kov | Costing | Cloud | $159/month |
| Aptean Process Manufacturing | Formulation and Recipe Management | Cloud or On-Premise | N/A |
| QT9 ERP | Regulatory Compliance | Cloud | N/A |
| Deacom Essentials | Batch Tracking | Cloud or On-Premise | $500 |
| QAD | Enterprises | Cloud or On-Premise | N/A |
| Acctivate Inventory Management | Traceability | Cloud or On-Premise | $5,000/year |
Pharmaceutical manufacturing software, on average, can cost from $150/month to $5,000/month. Mar-Kov is our most affordable choice, starting at $159/month. Other options, such as Datacor, are a bit more expensive, starting at $1,500. The cost typically depends on the number of users, the level of included support, and any additional features you require with your base package.