Mar-Kov
6 Reviews 4.5/5 ★ ★ ★ ★ ★A process manufacturing management system for chemical and batch manufacturing companies.
Product Overview
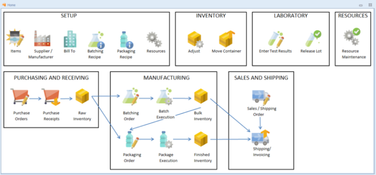
Mar-Kov is a specialized solution for the chemical industry, designed to enhance efficiency and accuracy in batch manufacturing processes. It integrates functionalities like formulation management, inventory control, and quality compliance in a single platform. The software employs technologies such as bar-coding and RFID for efficient, paperless solutions. It also interfaces directly with plant equipment, ensuring adherence to material and process quality standards.Pros
- Recalls and mock recalls are easy to perform
- Greatly increases the ability to fill orders
- Wide variety of reports
- Integrates with QuickBooks, Sage, ShipStation
Cons
- Slow response to support requests
- Can be inventory discrepancies
Target Market
Small to large companies in the food, chemical, fragrance, pharmaceutical, flavors, OTC, and cosmetics industries.Video Overview
Features
- Batch traceability
- Electronic batch recording
- Mass inventory storage system
Inventory Management/Warehousing
- Print the MSDSs and C of As required for a shipment automatically based on products and lots being shipped.
- Generate all labeling required for shipment of hazardous materials.
- Produce all paperwork required for international shipments, including those required for freight forwarding.
- Pack out into customer-defined container sizes that are integrated with the shipping process.
- Supports the use of shelf cans
- Ensures that mixed lots get sampled and retested
- Support for customer-defined labeling
- Web-based customer self-serve
- Order confirmation and advanced shipment notification using EDI or email technologies.
- Fine-tuned for container-level inventory tracking of chemicals, components, and finished goods
- Bolts onto any ERP system in synchronous or asynchronous mode
- Multiple warehouse/division support
- Direct weigh scale integration for weight verification during receiving, cycle counting, and shipping
- Complete audit trail of all transactions
- Easy to use context-sensitive ‘drill downs’ to any level of detail
- Many automated functions, including shipping document preparation
- Ability to view inventory snapshots at any time in the past
- Perform cycle counts and physicals without shutting down operations
- Wave picking for more efficient use of tow motor operators
- Designed to meet the stringent requirements of the FDA with 21 CFR part 11 compliance
- Designed to work in a ‘thin client’ environment, which lets the applications run on portable RF Pocket PCs or Pen tablets
- Optional QC/LIMS module controls item/product testing and subsequent quality code changes
Laboratory Information Management System
- Ensures that all testing is performed according to product specifications for both ingredients and bulk products.
- Coordinates the approval of materials where multiple samples and approvals are required.
- Prevents the use of unapproved or expired materials.
- Supports storage and retrieval of MSDS and Certificate of Analysis documents. These documents are directly linked to products and containers and can be retrieved online.
- Creates Certificates of Analysis from test results.
- Under strict management control, unapproved materials can be used pending their testing.
- Testing of in-process samples is fully integrated with other testing. The ability to enter adjustments is required for Batches.
- Gang Testing is supported.
Manufacturing Execution System
- Real-time consumption and creation of inventory.
- Computer-assisted weighing, blending, and packing out of manufacturing orders.
- Provides operators with accurate real-time inventory visibility and quality control status.
- Pre-weigh and co-mingle operations are performed using scales that are directly accessible to the software, enhancing speed and reliability.
- The software polices each step of the batching operation to ensure that all steps are performed as defined in the process specification.
- MSDS and HMIS information is displayed as required throughout.
- The software ensures that all equipment has been cleaned and sanitized before being allowed to be used.
- Provides an electronic batch sheet that the user may customize.
- A paperless system with data collected at the source eliminates the time and errors associated with manual data recording and entry
- Complete lot traceability with container-level tracking of source materials.
- Material consumption information is sent back to the host ERP in seconds. Ability to interface to ANY host system: Oracle, SAP, Batchmaster, MFG/PRO Data3, etc.
- Designed to meet the stringent requirements of the FDA with 21 CFR Part 11 Compliance
- Designed to work in a thin client environment, which lets the application run on portable Pocket PCs or Pen tablets
- Barcoding is used throughout to ensure accuracy
- Integrated in-process testing ensures that adjustments are correctly completed and retains details for review and analysis
- Automatically corrects quantities required based on the purity as required and determines the amount of filler required.
- Weight tolerances can be set at the ingredient level.
- Gang weighing is supported for increased efficiency.
- Filters ingredients by workstation based on security, pour size, and other criteria.
- Signatures for operator and checker can be individually configured for each operation.
Recipe Manager
- Automatically generates NSF/ANSI 455-3 and MoCRA-compliant electronic batch records.
- Secure audit trail of all recipe development.
- Define and enforce corporate standards for product and process specifications.
- Speeds up and enhances the consistency of recipe creation by using a library of predefined equipment and process definitions (an unlimited number of user-created definitions may be added).
- Integrates with the CTFA database for more accurate product definitions.
- Verifies that different parts of a recipe are consistent with each other. For example, it ensures that all ingredients defined in the formula are used in the process.
- Ensures that all sign-offs are present before a recipe can be released for production.
- Ingredients, specifications, and recipes may be shared amongst all users or specific groups.
- Implemented using S88 concepts and nomenclature.
- Designed specifically for cosmetic, pharmaceutical, and chemical industries.
- Eliminates errors commonly found in manually generated recipes.
Product Overview
Developer Overview
Related Products
User Reviews of Mar-Kov
Write a ReviewMar-Kov Review
Mar-Kov transformed our business. In just 24 hours, we were back in the black, and within months, it revolutionized how we manage operations. With Mar-Kov’s real-time, accurate data, we were able to streamline inventory, improve traceability, and eliminate errors in production. We’ve reduced costs, increased margins, and maximized production efficiency. Mar-Kov freed me up to focus on sales, and it allowed us to scale while ensuring consistency in our products. The transparency and historical data we get from Mar-Kov have been crucial for long-term success and sustainability.
Mar-Kov Review
Mar-Kov has significantly improved our operations. Its accurate, real-time traceability has helped us triple sales and win new customers, including big box retailers and multinational companies. We can now complete audits much faster, and auditors trust the system over paper records. We’ve reduced raw material inventory by 50%, cut costs by 5%, and increased margins by 10%. The system has also eliminated 1.5 full-time positions, saving over $100,000 annually. Our products are consistent, and Mar-Kov prevents mistakes like using the wrong ingredient. The personalized support and training made implementation smooth, with the ROI coming within 12 months.
Mar-Kov Review
Since going live with Mar-Kov’s full suite in 2022, we’ve seen unexpected benefits like better margins, cost savings, and 75% less admin time. Mar-Kov data confirmed we were retail-driven and helped us diversify into the industrial sector, identifying marketplace gaps. During the pandemic, Mar-Kov proved invaluable as consumer DIY demand and material prices skyrocketed, alongside supply chain shortages. It’s saved me a boatload of time – we can do our jobs better without leaving our desks and trust Mar-Kov’s data without double-checking. We’re now comfortable carrying 30% less inventory, which significantly improves cash flow. Mar-Kov’s reports also help us evaluate price changes and maintain margins. We know exactly which products are moving and what’s trending, and in the event of a complaint, we can track the exact lot or batch sent to customers. Additionally, Mar-Kov has securely protected our critical. formulations, ensuring quality consistency and preventing unauthorized changes. Mar-Kov has been 99% perfect for us, and compared to competitors, it’s 5 times less expensive, with no unnecessary over-engineering.
- Tobacco
- 11-50 employees
- Annual revenue $1M-$10M
Very detailed and very helpful in our day to day operations
This is a great product. It is very detailed and very helpful in our day to day operations. We use it to record inventory transactions, schedule maintenance, manufacture our product, ship our product all while maintaining full lot traceability on finished goods, raw materials and primary packaging. It is great for pulling reports of inventory movements, sales, expired product, demand and supply, available inventory, ect. Overall CMS is very helpful because it keeps us from having to try to remember everything. We’re a small business and it provides what we need in one place.
Pros
I like that we can perform a recall or mock recall with such ease and I love the Plan Tracing Set Viewer because it details our production plans and is multi faceted.
Cons
My least favorite think about CMS is the length of time it takes to resolve an issue. Support responds quickly but the resolution time is slower than we would like.
- Pharmaceuticals
- 11-50 employees
- Annual revenue $1M-$10M
Alex is very personable, patient, and a great source of information
We just got done with our second training session.
Mar-Kov provides a program that is tailored to our needs, with knowledgeable staff to help every step of the way. Joe from Applied Net Solutions has been incredibly helpful as well with helping find necessary hardware to make the implementation of the WMS a success.
What is happening now is they have presented us with a working copy of our WMS, which they continue to edit as we go along in order to fit better and better into our existing processes. Our trainer, Alex, is very personable, patient, and a great source of information.
So far, we love it.
- Food Products
- 11-50 employees
- Annual revenue $1M-$10M
There are several fundamental flaws with the program
We have faced an extreme amount of challenges with Mar Kov–many of the issues that we found when we first implemented the system two years ago are still issues that we deal with today. Our inventory is never correct, there are discrepancies that we cannot explain and when we bring it to Mar Kov they cannot explain it and quite frankly are very rude about it. The inventory load was recently fixed when we had been asking about it for two years. The system is very slow. It will over allot inventory at random which means that we have to triple check all of our waybills which creates a back up in our efficiency. There are a great many issues that we could discuss and frankly the IT support from Mar Kov is not there to help fix it. There is only so much we can do as a small company with no IT department to fix these issues. We pay quite a bit of money and were promised a great many things when we first implemented Mar Kov, unfortunately very few of those have been delivered nor have we had any satisfactory customer service to fix them.
Pros
While there are still several outstanding issues Mar Kov has allowed us to track our product and greatly increase our ability to fill orders. It helps facilitate extremely accurate communication between each department of our company. I also personally like the variety of reports we can pull from the system as it greatly helps with our sales and frequency planning.
Cons
There are several fundamental flaws with the program that make it almost impossible to work with at times. We deal with variable products and very unique products. When we purchased Mar Kov we were assured that these products could fit within the confines of Mar Kov's capabilities. I am starting to doubt that very much.