What is CAPA? A Guide to Corrective & Preventive Action
With CAPA, or Corrective and Preventive Actions, businesses can plan and conduct investigations into production failures, identify root causes, and execute improvements to their products and manufacturing processes. CAPA is one of the most critical drivers behind how businesses manage their overall quality management system.
In this CAPA guide, you’ll learn:
- What CAPA is
- The difference between corrective and preventive action
- How CAPA connects to broader quality management
- The 7 CAPA steps
- The 5 Root Cause Analysis (RCA) frameworks
- Who uses CAPA
- How technology is changing CAPA
What is CAPA?
CAPA stands for corrective and preventive actions. It is the formal process businesses use to investigate problems, fix their causes, and prevent them from happening again. CAPA helps manufacturers structure their improvement processes and identify root causes across product development, production, training, and compliance.
A strong CAPA process is designed to answer four main questions:
- What went wrong?
- Why did it happen?
- What are we doing to correct it?
- How do we prevent it from happening again?
CAPA is often required in quality-driven manufacturing environments, and is the backbone to ISO, FDA, and GMP compliance standards. The process is commonly managed through a Quality Management System (QMS) or dedicated CAPA Software.
Corrective Action (CA) vs Preventive Action (PA)
Corrective and preventive actions are closely related, but they solve different types of problems.
Corrective Action: These are actions taken after a quality issue has already happened. The goal of corrective actions is to eliminate the root cause so the issue cannot occur again.
A batch fails inspection due to an out-of-spec measurement. The team then investigates the cause and corrects the process to prevent further defects.
Preventive Action: These actions are taken before a quality issue has happened. The goal is to identify risks and put controls in place to reduce the likelihood of a problem occurring in the first place.
A supervisor notices the quality of materials from a supplier has decreased over the last few shipments. The team tightens incoming inspection requirements and reaches out to the supplier to prevent production issues.
CAPA’s Role in a Quality Management System (QMS)
CAPA is what makes a quality management system actually work in the real world. It is rarely a standalone process. Instead, it’s the mechanism that takes quality issues and turns them into controlled, documented improvements that strengthen your organization over time.
In practice, CAPA sits at the center of your quality process and is triggered whenever something flags a serious issue or recurring risk. A few examples of these quality events include:
- Nonconformances (internal defects or failed inspections)
- Audit findings (internal or external)
- Customer complaints and product returns
- Supplier issues (SCARs)
- Deviations and out-of-spec measurements
- Quality trend data (repeat defects, yield drops, rising scrap rates)
From there, CAPA follows a structured, repeatable framework and is supported by root cause analysis, ensuring the right fixes are implemented for the right reasons.
The 7 CAPA Steps
While the exact process varies by industry and the type of problem you are working to fix, the most common phases to complete a CAPA investigation is as follows:
1. Identify the Issue
- Document the quality event and define the problem clearly and in as much detail as possible. Ask questions like: What happened, where did it happen, when did it happen, and how often does this occur. Notify affected teams and customers.
2. Evaluate Risk and Severity
- Perform a risk evaluation to assess the severity and recurring impact of the issue. Determine if a formal CAPA investigation should take place and prepare a framework and timeline for resolution.
3. Perform Root Cause Analysis (RCA)
- Investigate what actually caused the nonconformity using one of the frameworks below. Focus on process breakdowns, equipment failures, and other contributing factors.
4. Develop the CAPA Plan
- After the investigation, determine the corrective and preventive actions needed. Assign owners to these actions, set timelines for updates, and document the required changes clearly.
5. Implement the Actions
- Next, execute the plan across the organization. Apply the required actions to the people, processes, and systems assigned to ensure it won’t happen again in the future.
6. Verify CAPA Effectiveness
- After implementing the plan, it is important to confirm if the solution was effective by monitoring inspection and audit results. If not resolved, further investigation must take place, and a new plan should be created.
7. Closure and Follow-up
- Once the problem is effectively resolved, formally close the CAPA and document the steps taken. Continue monitoring for recurrence through audits or routine follow-ups.
5 Root Cause Analysis Frameworks
Root cause analysis is the process of investigating why the problem occurred in the first place. As many manufacturers simply stop at the first visible symptom, it’s important to follow a consistent framework to diagnose where in the process the failure actually occurred.
Below are five of the most common root cause analysis frameworks used in CAPA investigations.
The 5 Whys:

The 5 Whys is a simple framework that asks “why?” repeatedly until the root cause becomes clear. This method works best for straightforward issues where the cause is likely tied to a breakdown in a single process, decision, or human action, rather than a complex, system-wide failure.
Fishbone Diagram (Ishikawa):
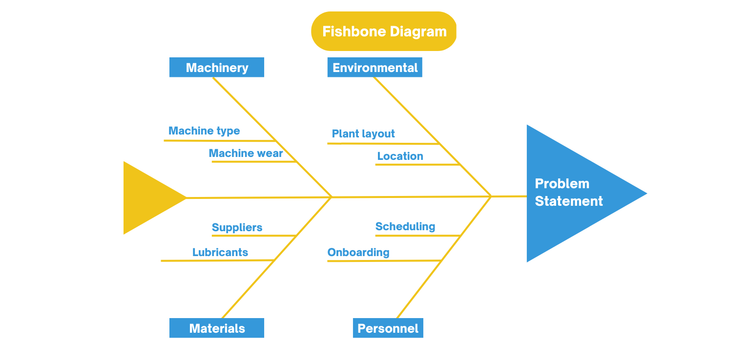
A Fishbone Diagram is used to visually break a problem into all its possible contributing causes (people, process, equipment, environment, measurement). It works by placing the problem at the “head” of the diagram and branching outward to the potential causes of the issue, helping teams systematically brainstorm rather than jumping to conclusions. This framework is used when problems involve multiple breakdowns spanning across departments.
Pareto Analysis:
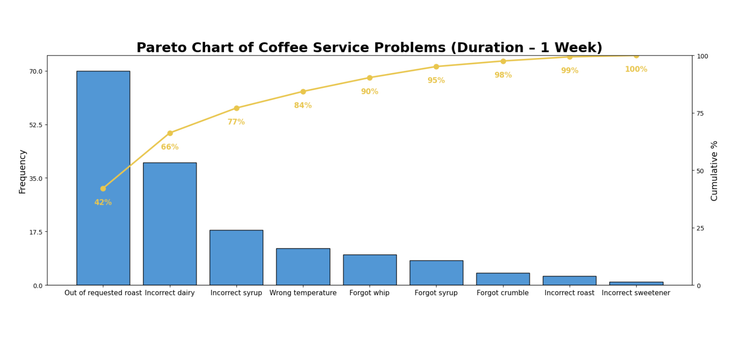
The Pareto Analysis framework is based on the idea that a small number of issues often drive the most impact. It helps teams identify which issues contribute the most to a problem by ranking failure types and defects by frequency or impact, so teams can focus on the “vital few.” The rankings are typically visualized using a Pareto chart and can only be used when there is enough data to spot clear patterns.
FMEA (Failure Modes & Effects Analysis):
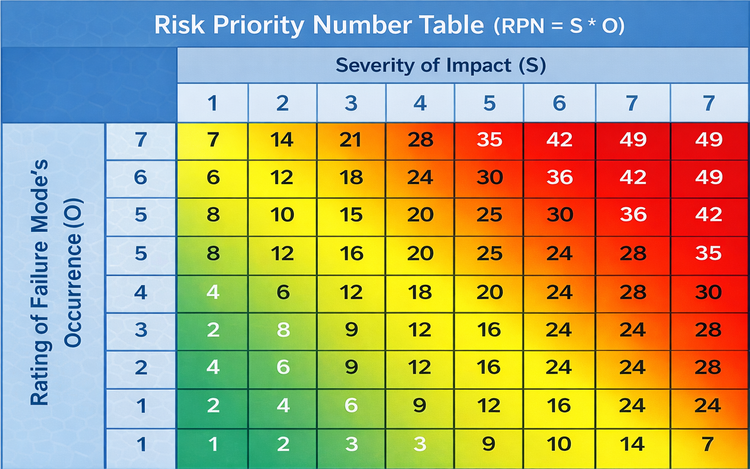
FMEA is a structured framework for proactively identifying potential failures in a process or system before they occur. It works by listing possible failures, what their impact would be, and what might cause them. Then those failures and risks are ranked by severity, likelihood of occurrence, and ease of detection in time. This framework is used when teams want to take preventive actions and catch issues early, rather than waiting for breakdowns to happen.
Fault Tree Analysis (FTA):
Fault Tree Analysis is a top-down framework used to identify how a singular failure could occur by mapping how different events, breakdowns, and conditions could lead to it. It starts with the issue you are investigating at the top of the tree and branches downward into contributing causes.
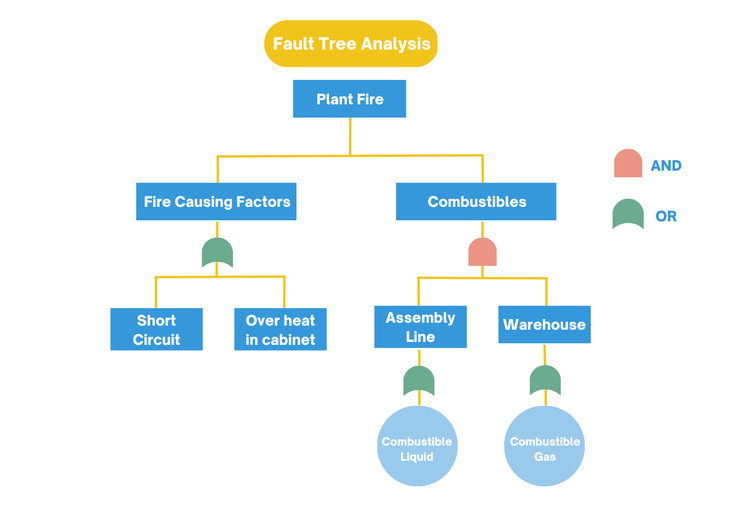
Then, using simple logic like “AND” “OR” relationships, it shows whether one failure alone could trigger the problem or whether multiple failures have to happen together. This framework is used when teams need to understand complex failures or isolate a single point of failure that traditional brainstorming might miss.
Who uses CAPA?
CAPA is used by any organization that needs to investigate problems, fix root causes, and prevent repeat issues. It’s most commonly used in regulated industries and quality-driven manufacturing environments where a formal process must be followed.
Regulated Industries:
- Medical Device
- Pharmaceuticals and Biotech
- Food and Beverage Manufacturing
- Aerospace and Defense
- Automotive Manufacturing
- Chemical Manufacturing
- Life Science Manufacturing
These industries need CAPA to comply with strict regulatory requirements and quality standards, including FDA, ISO 13485, AS9100, IATF 16949, and GMP regulations.
Quality Driven Industries:
- Electronics Manufacturing
- Industrial Manufacturing
- Contract Manufacturing
- Consumer Product (CPG) Manufacturing
- Packaging Manufacturing
These industries use CAPA because the cost of recurring quality issues is too high to manage informally. Even when CAPA is not strictly required, it’s adopted to reduce defects, prevent repeat failures, improve supplier quality, and protect customer trust.
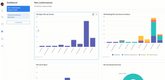
How Technology is Reshaping CAPA
Technology is revolutionizing how manufacturers manage CAPA by enabling faster investigations, more consistent workflows, and better tracking of quality data over time. Instead of relying on manual spreadsheets or disconnected tools, many manufacturers are achieving better transparency by moving to digital systems.
-
CAPA Software: These tools allow manufacturers to digitally track and manage documentation, nonconformities, root cause investigations, and corrective and preventive actions in a centralized platform. The software ensures that workflows comply with industry standards and that CAPA records and documents are audit-ready.
-
Mobile Audits: Mobile audit and inspection tools allow businesses to capture photos and supporting evidence directly from the shop floor. This speeds up reporting and reduces common documentation gaps that make it difficult to investigate process failures properly.
-
Automated Workflows: With automation in place, tasks and approvals can be routed to the right owner, and reminders can trigger when actions or compliance documentation are overdue. This improves the overall CAPA process by ensuring all steps are completed and preventing investigations from stalling.
-
Reporting and AI Analysis: With AI and advanced machine learning technology, quality and production data can be analyized to find insights and spot trends in your manufacturing processes. While these tools will not complete a deep RCA on their own, they can speed up the CAPA investigation phase and uncover systems issues that need to be addressed for long-term performance.
Quality Management Software for CAPA
Quality management software (QMS) is often the most effective approach to improve CAPA execution in a meaningful, long-term way. As production scales and quality requirements become more complex, software helps manufacturers standardize investigations, enforce consistent workflows, and maintain traceable CAPA records from initiation through closure.
Below are four quality management platforms we recommend that support CAPA for manufacturers across a wide range of industries and company sizes.
- Unifize is a quality management software for startups and growing manufacturers. It was built to support regulated manufacturing, product design, and the life science industries. It offers an easy-to-use interface with a collaborative workflow that turns quality conversations into audit-ready documentation.
- QT9 QMS is a quality management platform designed for midmarket and small businesses. It comes with over 25 integrated modules covering CAPA, supplier management, quality event management, and product design controls. It excels at managing CAPA for the life sciences, medical devices, and aerospace and defense manufacturers.
- ETQ Reliance is designed for enterprises and mid-market manufacturers operating globally. ETQ provides deep quality management control with support for EHS, compliance, and CAPA. It supports industries such as pharmaceuticals, high-tech electronics manufacturing, food and beverage, and medical devices.
- QAD eQMS is built for mid-sized and large organizations operating in manufacturing, automotive, healthcare, and pharmaceutical industries. It supports all quality management operations, including CAPA, document control, supplier management, and root cause analysis. QAD also provides industry-specific tools tailored to each unique industry it supports.