What is EQMS?
EQMS (enterprise quality management software) is a system that enables businesses to organize procedures, processes, and responsibilities related to best practices for achieving product goals and policies. Follow this guide to discover EQMS’s benefits and limitations and how it differs from QMS.
EQMS vs. QMS
Compared to standard quality management software, EQMS software helps larger businesses ensure quality and compliance throughout their supply chain.
QMS is either paper-based or uses basic electronic systems, whereas EQMS is a digital platform that leverages advanced technology to automate quality processes. EQMS can also handle larger volumes of data than QMS.
Who Uses EQMS?
EQMS is primarily used by large companies, especially those operating in highly regulated industries. Some common industries that use it include aerospace, manufacturing, automotive, healthcare, pharmaceuticals, government contracting, and food and beverage.
Healthcare is anticipated to emerge as an industry needing regulatory updates and innovation in medical devices. With the increased use of the Internet of Things (IoT), the demand for QMS solutions should increase over the coming years. Medical device manufacturers will benefit from using EQMS to maintain ISO 13485 compliance.
Key Features
Enterprise quality management software has introduced automated, standardized, and centralized processes for cross-functional collaboration. Certain functionalities are given in standard quality management processes, including quality planning, parts non-conformance, approval management, and complaint management.
Corrective and Preventive Action (CAPA)
Corrective and Preventive Action software (CAPA) is a continuous improvement tool that collects and analyzes key metrics to identify and eliminate product quality, process inefficiencies, and equipment issues. CAPA processes are used to correct nonconformances that may occur during production.
CAPA features within enterprise quality management software will alert you to these potential issues through risk assessments and root cause analysis, identifying the underlying problem with a product or process. By discovering the root cause, you’ll be able to resolve the issue easily, and actions can be taken to prevent the nonconformance from occurring again.
Audit Management
The right EQMS software can schedule, track, and execute audits and inspections–ensuring your documentation is always up-to-date, complete, and accurate. Audit management modules aim to implement best-practice audit planning, improve audit efficiency, drive continuous improvement, and enhance the visibility of results.
Audit management allows best practices to be implemented, such as creating audit programs and managing schedules and resources. These programs can be reused during future planning cycles, reducing time and labor costs. Integration with popular apps such as Outlook and Google Calendars can also improve audit efficiency, allowing your staff quick access to email notifications, dashboards, and shared calendars.
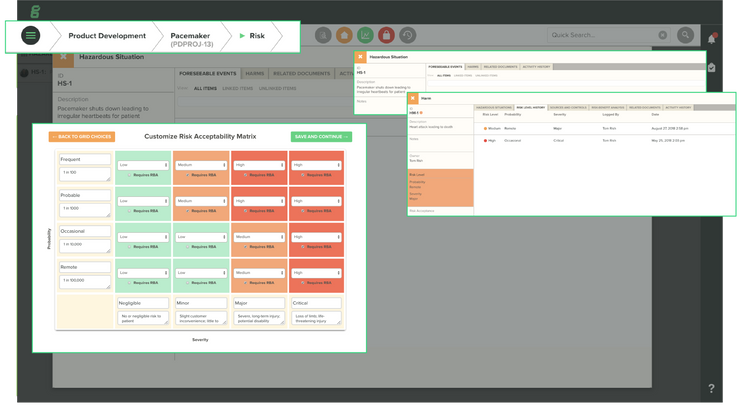
Risk Management
Manufacturers use risk management to identify hazards associated with their production processes. Additionally, they must deal with many compliance regulations that specify how to estimate, evaluate, and control these risks and monitor controls for effectiveness across the product lifecycle. Risk management modules within EQMS software allow you to create what-if scenarios to analyze potential costs related to quality exceptions reliably.
Risk differs from product to product, so you won’t want to limit your risk management processes to one method. EQMS solutions offer document management tools. By documenting your overall process, you can increase control of your project from concept through post-production–all while collecting details on your product line, machines, and overall project status.
Failure Mode and Effects Analysis (FMEA)
Failure modes and effects analysis refer to studying the consequences of the ways (or modes) in which something might fail. Failures include any errors or defects–usually ones that can/will affect the customer. FMEA features within EQMS software aim to decrease manufacturing costs by providing opportunities for preventive maintenance instead of reactive maintenance.
The following indicates a small sample of some of the benefits FMEA provides manufacturers:
- Define corrective actions
- Identify causes and effects
- Improve economic production
- Avoid defects
- Prevent failure
- Reduce product development time and costs
- Define and minimize risks
- Improve quality and reliability
Statistical Process Control (SPC)
Statistical process control (SPC) tools collect quality and performance data in real-time to identify product quality issues and process variations and take corrective action before extensive issues occur. These features can help reduce waste and downtime by measuring and controlling the underutilized resources in your organization, allowing for better decision-making regarding equipment issues.
SPC modules within enterprise quality management software monitor product quality control data and the performance of machines and other manufacturing tools. This data is displayed to shop floor personnel whenever product quality or machine performance falls outside predefined acceptable ranges. This data is typically presented via control charts, Pareto charts, or histograms.
Benefits
- Ensures Regulatory Compliance: Includes ISO 9001, Title 21 CFR Part 11, NERC, SSOP, HAACP, and more.
- Analyzes and Reduces Risk: Prevents errors, defects, unexpected service work, lawsuits, and recalls.
- Increases Customer Satisfaction: Ensures customers evaluate products for reliability, ease of use, and performance.
- Eliminates Inefficiency and Paper Waste: Electronic quality management systems help eliminate manual paper-based processes.
Limitations
While enterprise quality management software has several advantages (especially over basic QMS software options), it has certain disadvantages, primarily in cost. QAD EQMS typically starts at $25k per year.
The implementation process is also increasingly complex which can take time. However, as described above, these costs can be balanced out by the long-term benefits of comprehensive EQMS.
