Qualio QMS
11 Reviews 5/5 ★ ★ ★ ★ ★An eQMS for the life-sciences industry for ISO, FDA, and GxP compliance.
Product Overview
Qualio, a cloud-based eQMS, helps companies in the life sciences sector replace traditional paper-based quality management processes with digital solutions. The platform hosts a wide range of tools for effective supplier management and risk mitigation. It supports product development processes from a single source of truth to ensure compliance and audit readiness. Additionally, Qualio provides pre-configured templates for FDA and ISO compliance, and access to quality experts.Pros
- Meets FDA standards
- Good fit for first-time QMS
- Reduces physical paper trail
Cons
- Potentially complex setup
- Limited non-life science use
- Requires regular updates for best performance
Target Market
Organizations in the life sciences sector that need ISO and FDA compliance. Industries include medical devices, software as a medical device (SaMD), pharmaceuticals, cannabis, biotech and contract research organizations.Video Overview
Features
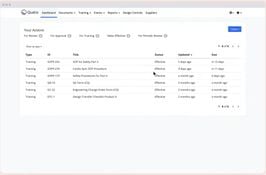
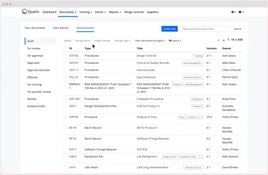
- Document Management: Centralize and digitize document control.
- Supplier Management: Categorize and manage suppliers with complete visibility.
- Design Control Management: Streamline product development.
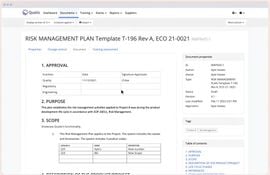
- Risk Management: Detect and mitigate risks, ensuring compliance.
- Training Management: Track and manage employee training.
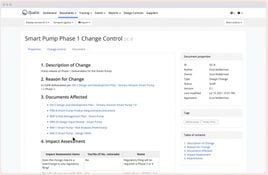
- Change Control Management: Efficiently manage and trace changes.
- Audit Management: Ensure audit readiness and compliance.
- CAPA & Non-Conformance Management: Automate response workflows.
- Analytics: Visualize quality metrics and trends.
Product Overview
Developer Overview
Related Products
User Reviews of Qualio QMS
Write a Review- Pharmaceuticals
- 1-10 employees
- Annual revenue $0-$1M
Good sized program for the price
As a dental device manufacturer, we were looking for a Quality Management System that could move us out of pen & paper into a streamlined system. We ended up finding the Qualio QMS program.
They were able to help us become regulated as a Class 2 dental device! So, ultimately we found that Qualio was able to meet our needs and their pricing fit well.
Pros
Good sized program for the price Had expereince with our indsutry
Qualio QMS Review
Qualio has been super helpful to us feeling like we have all the pieces in place for quality management system… to an FDA standard.
Qualio QMS Review
I would recommend Qualio to small companies implementing a quality system for the first time. Qualio helped us to reduce workload and paper trail.
Qualio QMS Review
Qualio enabled us to seamlessly work through the ISO 13485:2016 requirements and design errors out of our quality management system. One way Qualio achieves this is to ensure that changes flow through our system and are applied globally.
Qualio QMS Review
The validation documentation provided by Qualio was easy to understand and execute. I really like the in-app support chat function-it’s easy to get in touch with the Qualio team with questions or concerns and the team responds quickly.
Qualio QMS Review
It’s been a pleasure to work with Qualio.
Qualio QMS Review
The best thing I like about Qualio is how simple the documents are prepared for easy understanding by anyone who has no knowledge on what they’re reading, be it a policy or a basic learning document.
Qualio QMS Review
You can look at an SOP in Qualio, see the list of associated documents, and click hyperlinks to retrieve information immediately. That’s one feature I’m greatly enjoying.
Qualio QMS Review
I love Qualio… The most user-friendly system that I’ve ever used in my whole career.
Qualio QMS Review
Qualio has different modules all in one. No need to have different QMS software.
Qualio QMS Review
Qualio has helped us tremendously with the QMS and getting started.