The Best Medical Device Manufacturing Software
Medical device manufacturing software is a specialized tool used to design, produce, and manage the lifecycle of healthcare equipment. We tested and ranked the best systems that focus on compliance and corrective and preventative actions (CAPA).
- Open architecture for rapid integrations
- Multi-entity support
- Mobile accessibility
- Responsive customer service
- Quick record creation and retrieval through documents module
- Can be tailored to specific industries
- Free trial available
- New eCommerce additions
- Low total cost of ownership
We used our software review methodology to rank the top options on the market today, including Acumatica, QAD EQMS, and xTuple.
- Acumatica: Best Overall
- QAD Adaptive: Best for Enterprises
- Cetec ERP: Best for Medical Device Startups
- DELMIAWorks: Strong Manufacturing Intelligence
- ECI M1: Best Make-to-Order Support
- Dynamics 365 Business Central: Best Product Ecosystem
- Infor CloudSuite: Best for Complex Manufacturing
Acumatica - Best Overall
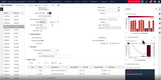
Acumatica ‘s compliance functionality integrates with its cloud-based ERP system, allowing for real-time data collection, automated reporting, and secure access to critical information. The system enables you to maintain accurate records, track product lifecycles, and ensure adherence to FDA guidelines and ISO standards.
Before manufacturing starts, Acumatica verifies the approved supplier lists and quality certifications to ensure the right materials are used. Then, during production, the system enforces in-process quality checks, flagging potential issues before they reach the final stage.
Acumatica records every step of production, from raw material intake to final product inspection. If any error occurs throughout the process, this ERP triggers corrective and preventative actions (CAPA) workflows, logging all changes in an audit trail for review. The software can also generate compliance reports on demand for audits and regulatory submissions.
QAD Adaptive - Best for Enterprises
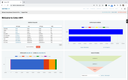
QAD Adaptive ERP is best for mid-sized to large medical device manufacturers operating at scale in heavily regulated industries. These include a document control system that gives teams across the enterprise access to the latest revisions and engineering changes. This is crucial for maintaining compliance with FDA and ISO regulations, which demand meticulous traceability throughout the device lifecycle.
The software is deeply integrated with QAD’s electronic quality management system, which comes with corrective and preventive actions (CAPA), supplier quality management, and continuous improvement modules. These features enable manufacturers to proactively manage quality across the entire supply chain, from raw materials to finished products.
By having a single integrated platform to manage both production and quality, QAD is particularly well-suited for enterprises and mid-market medical device manufacturers operating across multiple production lines or locations.
Cetec ERP - Best for Medical Device Startups
Cetec ERP is a cloud-based software built for growing and mid-market medical device manufacturers looking to replace spreadsheets and manual quality tracking. It combines core ERP functionality, such as inventory, production, and accounting, with built-in quality management and traceability to provide a one-stop shop for start-up manufacturers.
Because Cetec ERP has native quality control, start-ups can easily remain compliant with regulatory standards such as ISO 13485, FDA, and CFR Part 11. Quality teams can manage document revisions, nonconformances, corrective actions, and product inspections all from one place. Beyond quality control, Cetec also offers tools for production and inventory management. It provides lot and serial-based inventory tracking, while electronic job travelers keep production running smoothly and capture real-time updates from the shop floor.
Cetec ERP is best suited for startups and growing medical device manufacturers that value its affordable, transparent pricing model. With costs starting at $50 per user per month, smaller teams can implement a full ERP without the enterprise-level overhead. That said, larger, more complex global manufacturers may prefer systems with deeper enterprise functionality, such as QAD Adaptive or Oracle Fusion Cloud.
DELMIAWorks - Strong Manufacturing Intelligence
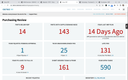
DELMIAWorks’ RealTime Process Monitoring System offers the oversight required in ISO- and FDA-regulated environments. The system connects directly to equipment, supporting legacy machines through programmable logic controllers (PLCs) and retrofit sensors. Live data streams then track parameters like cycle times and temperature stability–variables affecting product quality and regulatory compliance.
Additionally, DELMIAWorks auto-calculates overall equipment effectiveness (OEE). This can help you pinpoint performance issues like underperforming machines or drift in validated processes. For example, alarms are triggered instantly if equipment breaches preset tolerances for factors like pressure. This medical device manufacturing ERP then alerts your team via email or SMS for swift corrective action.
This data doesn’t just stay on the shop floor, though. DELMIAWorks stores historical records by device type, lot number, or operator. This supports device history record (DHR) requirements and potential ISO 13485 audits. Plus, the built-in SPC module maps real-time process data against your quality metrics and tracks how subtle fluctuations impact scrap rates. That way, you can proactively adjust machines before defects ever occur, minimizing the risk of nonconforming products.
DELMIAWorks starts at over $25,000 annually, or about $250/user/month with a five-user minimum. This pricing marks it a strong fit for mid-sized medical manufacturers.
Learn more about DELMIAWorks in our full review.
ECI M1 - Best Make-to-Order Support
ECI M1 provides robust support for make-to-order manufacturing processes, a common requirement in the medical device industry. Its specialized job costing, scheduling, and tracking tools enable manufacturers to manage custom orders efficiently and precisely. The software’s ability to provide real-time data on job status, material availability, and production timelines helps businesses minimize lead times and improve customer satisfaction.
The system’s integrated CRM and ERP functionalities further streamline the make-to-order process, from initial customer inquiry through production and delivery. This seamless integration ensures that all departments access the same up-to-date information, facilitating better communication and coordination across the organization. ECI M1’s focus on the specific needs of make-to-order manufacturers makes it the top choice for medical device companies specializing in custom products.
Dynamics 365 Business Central - Best Product Ecosystem
Dynamics 365 Business Central offers strong integrations within the Microsoft ecosystem, including Office 365, Outlook, and Azure. This integration facilitates smooth workflows and communication, allowing manufacturers to leverage their existing Microsoft infrastructure. Additionally, its manufacturing module handles complex BOMs, ensures product quality and traceability, and supports FDA compliance, ISO 13485 standards, and other global regulatory requirements.
Additionally, Business Central provides tools for documentation, audit trails, and compliance tracking, ensuring manufacturers can maintain and demonstrate compliance effectively. Business Central’s supply planning tools help maintain inventory levels, forecast demand, and plan material purchases, essential for uninterrupted production and delivery of medical devices.
Infor CloudSuite - Best for Complex Manufacturing
Infor CloudSuite Industrial is specifically designed to handle the complexities of manufacturing processes typical in the medical device industry. Its advanced planning and scheduling capabilities, detailed traceability, and quality management features ensure manufacturers can maintain high quality and compliance standards. Additionally, we found CloudSuite Industrial efficient at managing complex product configurations.
The platform’s industry-specific functionalities, including support for FDA compliance and medical device reporting, make it a comprehensive solution. CloudSuite Industrial’s focus on addressing the unique challenges of complex manufacturing processes, combined with its cloud-based deployment options, ensures that businesses have the agility and control needed to succeed in this demanding industry.
What Is Medical Device Manufacturing Software?
Medical device manufacturing software are digital systems designed to streamline and optimize the production of implantable devices, diagnostic equipment, wearables, and surgical instruments. Medical device software typically includes manufacturing Enterprise Resource Planning (ERP) components tailored to the industry, such as quality tracking and regulatory compliance features.
Medical devices are often subject to regulatory agencies such as the U.S. Food and Drug Administration (FDA), or International Organization for Standardization (ISO). Specialized manufacturing systems aim to ease compliance and improve profits.
Key Features
Medical device manufacturing software covers a wide range of features and benefits. A few of the most important features include:
- Regulatory Compliance Management: Supports adherence to FDA and EU MDR regulations for product identification and ensures electronic records and signatures meet all requirements.
- Quality Management: Captures deviations and defects in the manufacturing process for quality control.
- Product Lifecycle Management: Used to manage a product’s entire lifecycle from initial concept and design, through engineering, manufacturing, and end-of-life.
- Traceability and Tracking: Ensures complete product visibility for regulatory compliance, supporting serialization and recall management.
- Device History Record (DHR) Automation: Automates and manages medical device production history records, reducing paper-based tracking.
- Expiration Date and Shelf-Life Tracking: Ensures components and final products are sold before expiration.
Benefits
Medical device manufacturing software solutions offer a comprehensive approach to enterprise data information systems. Manufacturing ERP software seeks to link financial and operational “back-office” functionalities with operational and “customer-facing” technology.
An ERP system’s base benefits include:
- More efficient workflows,
- Visibility into processes for continuous improvement,
- Prevention of data re-entry,
- Robust reporting for enhanced operational and financial visibility,
- Improved data security, and
- Facilitation of collaboration opportunities.
Compliance With Quality Control Standards
Your medical device manufacturing business must deal with the impact of operating in a highly regulated industry daily in many different ways. The FDA, of course, not only governs food and pharmaceuticals but also has specific standards for device manufacturers. ISO 13485 is a certification developed explicitly for medical device manufacturers, which contains specific requirements for manufacturing, installation, and servicing. ISO 13485 calls for:
- Implementing a quality management system,
- Risk management,
- Statutory and regulatory compliance,
- Process validation, and
- Effective product traceability and recall systems.
Ultimately, compliance is a matter of quality control management and reporting standards. It can include planning, inspections, document tracking, product specifications, deviation tracking, parts approval tracking, and testing.
Traceability is also a significant factor. Inventory management tracks each SKU, serial number, and lot number, allowing you to identify where and when each item was manufactured.
Research and Development
For many medical device manufacturers, a product’s life starts in the research and development stage. You can spend many hours designing and engineering the products, testing, and conducting market research. This software helps track costs and identify where improvements can be made in the R&D process to ensure you have the most methods in place.
How to Choose Medical Device Manufacturing Software?
Selecting medical device manufacturing software requires more than just comparing feature lists. It involves evaluating whether a system aligns with your regulatory requirements and the complexity of your production workflows that generic manufacturing software can’t provide.
The following considerations can help guide the decision-making process and ensure the software you choose supports your operations.
Regulatory Compliance & Quality Tracking
Medical device manufacturers should start by evaluating whether a system is designed for a regulated environment. This includes understanding how the software supports controlled processes, documentation standards, and traceability requirements expected by FDA and ISO regulations. Software built for medical device manufacturing should enforce this accountability by default rather than bolting on compliance afterward.
Software Validation
Software validation is not a one-time task completed at implementation. Software buyers should assess how a platform handles ongoing regulatory validation as updates are applied and configurations change. A well-suited system should support ongoing compliance with 21 CFR Part 11 for electronic records and signatures.
Manufacturing Mode & Production Fit
Medical device manufacturing spans a wide range of production models, from low-volume, highly complex products to high-volume, repeatable operations. Buyers should evaluate how the software supports their specific manufacturing approach, including how work orders are executed, inspections are performed, and quality checks are enforced. Software should adapt to the existing production processes rather than forcing manufacturers to change how products are built.
Software Scalability & Business Growth
Software that works today may not work tomorrow. As medical device companies grow, production volumes, product portfolios, and regulatory risks increase. Buyers should consider whether a system can support future growth plans without introducing new compliance risks or operational inefficiencies. The right platform helps organizations scale while maintaining process control, visibility, and consistency across multiple teams and manufacturing lines.
Implementation, Training & Support
Medical device manufacturing software becomes a competitive advantage and long-term operational foundation. Beyond licensing costs, buyers should consider how the system fits into their broader technology environment and the internal resources required to maintain it over time. Implementation effort, integration complexity, and the impact of future updates all influence long-term value. A good fit software reduces operational friction rather than becoming another compliance headache.