The Best Pharmaceutical Distribution Software
Pharmaceutical distribution software supports the management, tracking, and delivery of pharma products across the entire supply chain to ensure compliance with the DSCSA. We tested various systems for pharmaceutical wholesalers and drug distribution companies of all sizes.
- Uses Champion AI to turn ERP into a proactive "system of action"
- Accelerated implementations (as little as 90 days)
- Integrated with QAD EQMS for quality management
- Provides batch tools through the order manager to track process workflows
- Integrates directly with QuickBooks for easy data migration
- Can handle millions of SKUs for scalability
- Multi-currency support
- Traceability (lot tracking)
- Customizable based on industry
We ranked the top systems based on their support for specific industry sizes, from small to global distribution, and their focus on areas such as wholesale or specialty pharmaceuticals.
Follow this guide to determine which system is the best in your industry:
| Software | Best For | Starting Pricing Per Month | Deployment Options |
|---|---|---|---|
| QAD Adaptive | Global Operations | $4,000 - $12,000 | Cloud and On-Premise |
| Acctivate | Specialty | $400 - $1,000 | Cloud or On-Premise |
| Blue Link | Wholesale | $500 - $3,500 | Cloud and On-Premise |
| NetSuite | Large Corporations | $3,000 - $8,500 | Cloud |
| ProfitPoint by TurningPoint Systems | Small to Mid-size Distributors | N/A | Cloud |
| Infor CloudSuite Distribution | Enterprise Distributors | $3,000 - $10,000+ | Cloud |
- QAD Adaptive: Best for Global Pharmaceutical Distributors
- Acctivate: Best for Specialty Distributors
- Blue Link: Best for Pharmaceutical Wholesalers
- NetSuite: Best Compliance Tools
- ProfitPoint ERP: Best for Small Distributors
- Infor CloudSuite Distribution: Best for Enterprise Distributors
QAD Adaptive - Best for Global Pharmaceutical Distributors
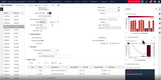
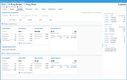
In an industry where regulatory adherence is paramount, QAD Adaptive is one of the few platforms with a global footprint that helps with compliance across multiple jurisdictions and trade zones. The software features restricted party screening, which is a compliance screening tool with tailored workflows and real-time monitoring.
This tool can automatically screen sale orders for restricted parties, embargoed countries, and other compliance-related criteria, ensuring adherence to complex and frequently changing international trade regulations without requiring manual intervention. Ensuring that every shipment is vetted in real time lets you confidently proceed with orders, knowing they meet all legal obligations. A green checkmark will indicate if a customer or order passed compliance screening.
For example, a distributor shipping prescription drugs to Europe must comply with regulations like the EU’s Falsified Medicines Directive. The compliance screening tool automatically checks for embargoed destinations and ensures compliance with each region’s unique requirements. If a shipment contains a controlled opioid, QAD flags it for additional checks based on the recipient’s country, preventing costly shipment rejections and delayed deliveries to customers and patients.
Once the tool verifies that the order passes all compliance checks, it’s automatically released to ensure timely shipments. Built-in workflows streamline picking and shipping, and your task list displays any pending steps before shipment. Shipping documents are automatically created based on your shipment type, which can help remove bottlenecks and shipping delays.
Acctivate - Best for Specialty Distributors
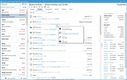
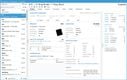
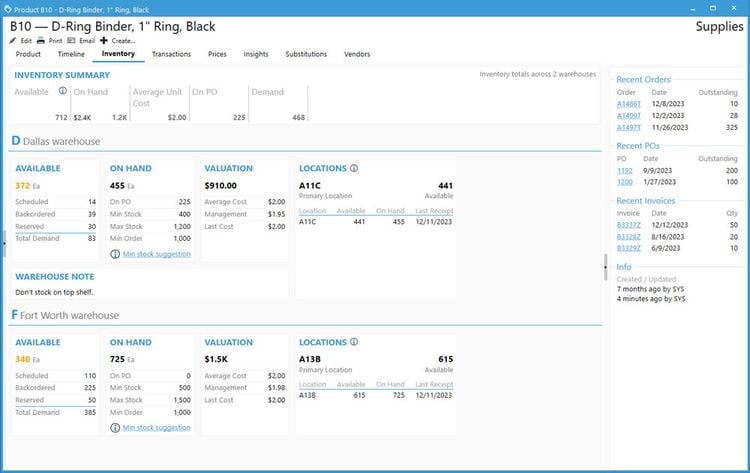
Acctivate enables you to create and manage multiple physical, virtual, and third-party warehouses within a single system. This is important when dealing with high-value, low-volume medications with specialized handling and storage needs, making monitoring sensitive items across different facilities easier. The system provides real-time visibility into inventory levels across your organization so you can easily track stock at cold storage areas, shipping containers, and 3PL providers. This transparency helps prevent stockouts and enhances the ability to monitor temperature-sensitive medications like biologics, oncology treatments, insulin, and specialty infusions.
Acctivate can automatically determine the optimal warehouse to fulfill each order based on inventory availability and proximity to the customer. This can minimize shipping times so customers can receive their life-saving medications without delay. For example, the system can track which oncology medications are stored in which locations and choose the best warehouse to fulfill each order, considering location, stock levels, and storage conditions like temperature. The system enables you to add warehouses, whether you create a new one or copy an existing one, making it adaptable to changing business needs. Additionally, moving products between warehouses is straightforward, with inventory transfers recorded to maintain accurate stock levels.
To ensure that inventory remains optimal, Acctivate provides notifications for restocking. You can quickly generate purchase orders through the reorder window based on customized alerts tied to each warehouse’s stocking requirements. This helps prevent stockouts and ensures you always have essential medications in stock when needed. Acctivate can also show cost differences in your products between different warehouses. Understanding the cost of goods sold as products move between locations enables you to optimize pricing and improve profitability while maintaining service quality.
While other ERP systems, like Odoo, offer strong multi-warehouse management, they don’t provide the same level of industry-specific features that specialty pharmaceutical distributors require, such as lot and expiration date tracking and advanced compliance management.
Read our full review of Acctivate to learn more.
Blue Link - Best for Pharmaceutical Wholesalers
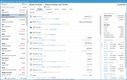
Blue Link has a controlled substance ordering system (CSOS) that can help you maintain compliance with the DEA requirements for ordering, tracking, and reporting on controlled substances. This system eliminates the need for the DEA form 222 by digitalizing the entire process, allowing you to submit electronic orders and speed up processing times. This is useful if you’re a wholesaler managing high volumes of standard prescription drugs, ensuring you process orders correctly while reducing the risk of regulatory violations.
The system automatically validates license information for the buyer and seller, ensuring that transactions can’t be processed without proper DEA authorization. It also uses digital certificates and electronic signatures to authenticate users and verify orders, enhancing security and reducing the risk of fraudulent transactions.
For a wholesaler supplying over 50 pharmacies, manually checking DEA licenses for each transaction could lead to delays and errors, risking compliance issues. Blue Link’s CSOS automates real-time license verification for each order, meaning you’ll never send out shipments without proper authorization. This ensures your pharmacies receive their orders quickly without risking legal penalties.
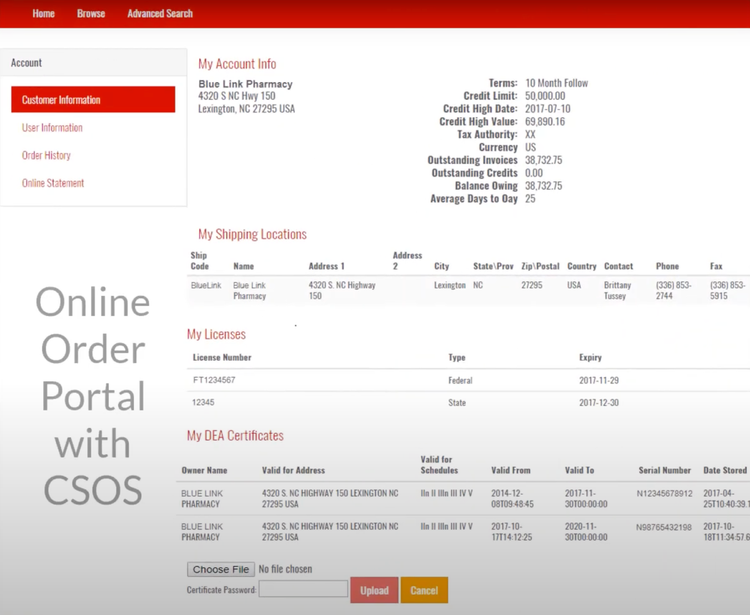
CSOS integrates with BlueLink’s compliance tools, like transaction history reporting and automation of reports and consolidated orders (ARCOS), to maintain a complete record of all transactions. This feature can help you monitor suspicious order patterns and prevent illegal distribution.
While Blue Link is ideal for wholesalers, it’s not as strong for specialty pharmaceutical distribution. That’s because it lacks advanced capabilities for handling temperature-sensitive products and environmental monitoring features that systems like Acctivate are more equipped to manage.
Read our full review of Blue Link to learn more.
NetSuite - Best Compliance Tools
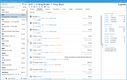
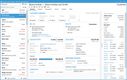
NetSuite offers a Life Sciences Edition with advanced compliance tracking for mid-size to large pharmaceutical distributors. One of its impressive features is the comprehensive audit trail functionality, which logs every user interaction and change made within the system. This lets you automate your recall process by linking product batches to customer orders, ensuring immediate and accurate action during compliance checks.
Large distributors must adhere to strict regulatory requirements, all while managing multiple facilities, higher transaction volumes, and complex supply chains. This creates a need for compliance tools to support advanced tracking, automated recall processes, and detailed audit trails.
The Compliance 360 dashboard has key performance indicators highlighting customer records that were searched, edited, exported, and deleted. On the home page, you can see which files were changed and who accessed them. An activity log also lets you view all recent activity by searching for a specific customer, date range, action, or user role.
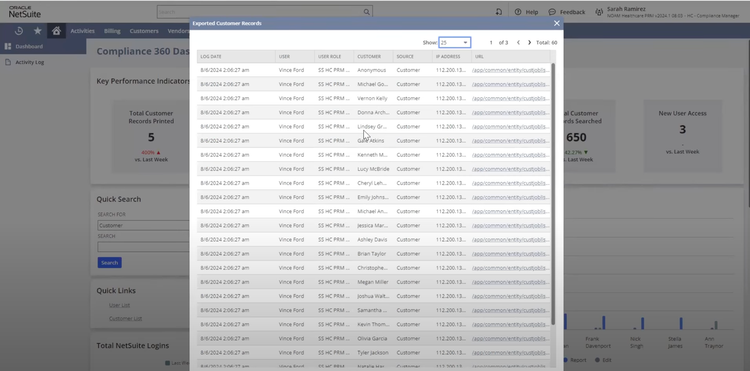
NetSuite’s Compliance 360 dashboard works with FDA guidelines and international regulations, such as GDPR and EU GDP standards. This capability allows large distributors to adhere to varying compliance requirements without switching systems or implementing complex integrations.
Read more about NetSuite in our full review.
ProfitPoint ERP - Best for Small Distributors
Compliance requirements like the DSCSA can be challenging for smaller distributors who may not have the same resources as larger companies. ProfitPoint by TurningPoint Systems Electronic Product Code Information Services (EPCIS) functionality is a compliance and data-sharing solution that helps you meet the FDA’s Drug Supply Chain Security Act (DSCSA) requirements. The easy-to-use system simplifies compliance processes, making it a practical and manageable choice for small businesses with less than 100 employees.
Their ProfitPoint software automates the track-and-trace requirements specified by the DSCSA, allowing you to track inventory from receipt to shipment seamlessly. It can manage lot and serial numbers, as well as expiration dates, to maintain transparency in the distribution process. This visibility into the location and status can help prevent loss, theft, and counterfeit drugs.
Additionally, the system can generate and manage essential compliance documents, including Transaction Information (TI), Transaction History (TH), and Transaction Statement (TS). By integrating with ERP systems, it streamlines key processes such as packing, shipping, receiving, and reporting. EPCIS facilitates an automated exchange of key documents (like EPCIS XML and EDI 856 ASN) between trading partners. This can help reduce manual processes to ensure quick and accurate communication with suppliers and customers, which is essential for small distributors with limited resources.
Unlike other small distribution software, ProfitPoint is designed specifically for the pharmaceutical distribution industry, providing tools that address its regulatory requirements and challenges.
Infor CloudSuite Distribution - Best for Enterprise Distributors
Infor CloudSuite Distribution is cloud-based pharmaceutical distribution software built for enterprise-scale operations. It delivers tools to manage complex supply chains, high transaction volumes, and multiple warehouses at scale.
Its standout capability is managing intricate distribution operations across multi-warehouse supply chains. Enterprise pharma distributors can monitor inventory in real-time and forecast demand and availability across multiple sites. For example, a distributor with dozens of facilities or retail stores can use the system to balance stock between locations to avoid shortages if demand surges in a specific region. And with built-in lot, serial, and expiration tracking, Infor CloudSuite Distribution supports compliant pharmaceutical inventory control, while full FDA, DSCSA, and global compliance generally requires complementary traceability solutions.
Because it is delivered as a cloud-based SaaS platform, CloudSuite Distribution benefits from continuous updates and reduced IT overhead compared to legacy systems. The tradeoff is that implementations can be complex, especially for companies migrating off older ERPs. Pricing reflects its enterprise scope, scaling by company size, and deployment needs, so smaller distributors may find it more than they need. But for organizations managing thousands of SKUs and enterprise logistics, CloudSuite Distribution provides a scalable, compliance-ready platform.
Systems We Also Like
SAP Business One has a lot and batch tracking feature designed to help better manage inventory movement. Each lot and batch is given a unique identifier, making it easier to trace products from production to delivery. The system can also track expiration dates, ensuring expired products are not distributed.
S2K Enterprise by VAI Systems is another solid option for pharma distributors. It comes equipped with DSCSA compliance through item sterilization and pedigree lot tracking, ensuring items are tracked through the entire supply chain. The ERP includes other integrated modules, including inventory, sales, order management, and analytics, making it well-suited for mid to large-sized companies.
What is Pharmaceutical Distribution Software?
Pharmaceutical distribution software is designed to ease the movement of regulated medications and controlled substances along the supply chain. It offers key features like inventory management, order processing, warehouse management, and regulatory compliance.
Unlike generic distribution ERP systems, pharmaceutical software takes regulations set by the U.S. Drug Supply Chain Security Act (DSCSA), Food and Drug Administration (FDA), Drug Enforcement Agency (DEA), and international regulatory agencies into account to remain compliant during the packaging and shipping process.
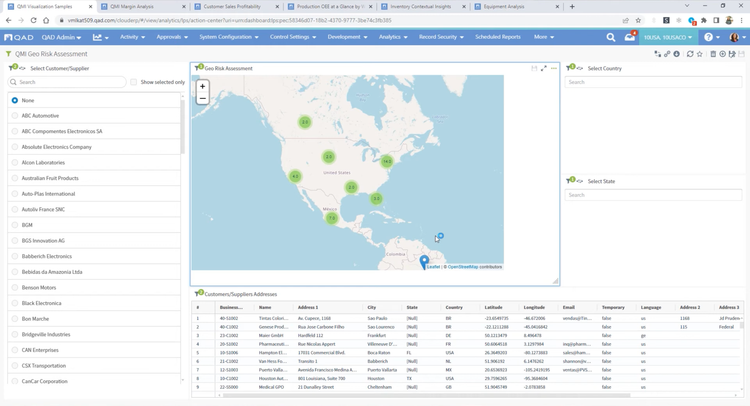
Key Features and Benefits
- Traceability: Helps you maintain detailed records of a product’s journey through the supply chain and quickly respond to any safety issues or recalls.
- Inventory Management: Tracks stock levels across multiple warehouses and offers lot traceability and serialization support for DSCSA compliance.
- Regulatory Compliance: Supports FDA, DEA, and DSCSA requirements and has built-in compliance checks for controlled substances and prescriptions.
- FIFO/LIFO/FEFO Tools: Utilizes first-in, first-out sorting, along with popular variations, to ensure medication moves in and out of your pharma facility before it has a chance to expire.
- Warehouse Management: Controls where your medical supplies are stored and distributed and streamlines retrieval operations when new orders or refills are placed.
- Electronic Signature Capture: Records signatures electronically to establish a clear chain of custody of all medications in your possession.
- Recall Management: Uses traceability tools to manage any recalls of pharmaceutical products, medications, drugs, and controlled substances.
- EDI Applications: Electronic Data Interchange tools keep documents compatible across different agencies. Standardize shipping statuses, invoices, purchase orders, inventory documents, and customs information for your B2B manufacturers and suppliers.
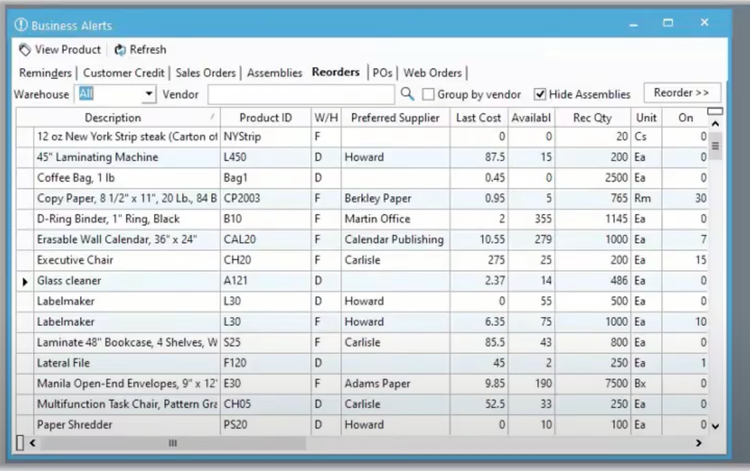
Common Challenges
Pharmaceutical distribution software can help solve these common challenges you may face daily:
- Quality and Safety Concerns: Medications can go bad when not in a temperature-controlled environment. Cold-chain management in your software can ensure that your products remain at optimal temperatures throughout the supply chain.
- Maintaining Compliance: With the pharmaceutical industry being highly regulated, it can be difficult to keep up with constantly changing regulations. Pharmaceutical software follows GDP guidelines and often complies with FDA and DEA requirements.
- Inventory Optimization: Balancing the need to avoid stockouts while preventing overstock and waste can be challenging. Many distribution systems automatically reorder low stock for you, so you don’t need to worry about running out of product or overstocking.
Pricing Guide
Pharmaceutical distribution software prices vary depending on the size of your industry, the exact features you’re looking for, and ongoing maintenance. Here’s a general overview of what you can expect to pay per year for a system based on your company size, with example products:
| Tier | Company Size | Average Yearly Cost | Examples |
|---|---|---|---|
| Low-Tier ERP | 1–50 employees | $5,000–$30,000 per year | Odoo, ERPNext, Intacct Distribution, QuickBooks Enterprise, DigitBridge |
| Mid-Tier ERP | 50–250 employees | $30,000–$150,000 per year | Acumatica, Microsoft Dynamics 365 Business Central, NetSuite, Odoo, SAP Business One |
| High-Tier ERP | 250–1,000 employees | $150,000–$500,000 per year | NetSuite, SAP Business ByDesign, Epicor ERP |
| Enterprise ERP | 1,000+ employees | $500,000+ per year | SAP S/4HANA, Oracle Fusion Cloud ERP, Microsoft Dynamics 365 Finance & Operations, Workday, IFS Cloud |
Whichever system you choose, you’ll likely need to contact the developer to receive a customized quote. The table below will show you a general idea of what the subscription fees would cost for each system.
| Software | Pricing Model | Subscription Fees |
|---|---|---|
| QAD Adaptive | Subscription-Based | Starts at $4,000/month |
| Acctivate | Subscription-Based | Starts at $400/month |
| Blue Link | Subscription-Based | Starts at $500/user/month |
| NetSuite | Subscription-Based | Starts at $3,000/month |
| TurningPoint Systems | N/A | N/A |
| Infor CloudSuite Distribution | Subscription-Based | Starts at $3,000/month |
Controlled Substance Compliance
Since 2013, the Drug Supply Chain Security Act (DSCSA) has been a national framework for securing the U.S. pharmaceutical supply chain. The primary goal of DSCSA is to prevent counterfeit, stolen, contaminated, or otherwise illegitimate drugs from entering distribution channels by requiring full traceability across supply chain partners.
Implementation was rolled out in phases, starting with enhanced drug distribution security requirements that required electronic, interoperable, package-level tracing. For pharmaceutical distributors, 3PLs, manufacturers, and dispensers, DSCSA compliance is not just a regulatory concern; it reshapes how supply chains and daily operations are controlled.
What the DSCSA Requires Today
Under the Drug Supply Chain Security Act (DSCSA), pharmaceutical distribution partners must:
- Exchange Transaction Information (TI), Transaction Statements (TS), and historical Transaction History (TH) with each transfer of ownership.
- Maintain serialized product identifiers, including GTIN, serial number, lot number, and expiration dates for all products.
- Verify product identifiers match what trading partners provide.
- Investigate and quarantine suspect or illegitimate products.
- Maintain records for at least 6 years for regulatory review.
- Enable secure, interoperable electronic data exchange across all trading partners.
The shift to package-level serialization means distributors must now track inventory at the unit level rather than just by lot. This significantly increased data volume, complexity, and integration requirements across internal software and trading partner systems.
How DSCSA Impacts Pharmaceutical Distribution Operations
Distributors must now support:
- Serialized inventory management across all locations
- Electronic Product Code Information Services (EPCIS) data exchange
- Integration with Verification Router Services (VRS) for returns validation
- Real-time interoperability with trading partners (manufacturers & upstream suppliers)
- Automated exception handling for mismatches or invalid serial numbers
- Audit trails that document every transaction and verification event
Without purpose-built pharmaceutical distribution software, these processes become very manual, prone to errors, and difficult to scale. For midmarket and enterprise distributors handling high volumes, a dedicated industry-specific software like a distribution ERP or WMS is required to operate efficiently.